Speak directly to the analyst to clarify any post sales queries you may have.
A concise strategic orientation describing how integrated scientific capabilities and regulatory readiness define successful small molecule CDMO partnerships
The lifecycle of small molecules within the contract development and manufacturing organization (CDMO) ecosystem is undergoing substantive evolution driven by scientific complexity, regulatory rigor, and shifting supply chain expectations. Modern small molecule development demands an integrated approach that combines analytical services, API manufacturing, formulation and packaging, and sophisticated process development. Across these domains, organizations require partners capable of bridging early-stage discovery through commercial delivery while maintaining traceability, reproducibility, and regulatory compliance.As development programs progress from preclinical validation to later clinical stages, the emphasis shifts from exploratory synthesis and route screening to robust process optimization and scalable technology transfer. This trajectory necessitates CDMOs that not only offer depth in catalytic, reagent, and solvent screening during route selection, but also the operational excellence to translate optimized chemistry into reproducible, commercial-scale manufacturing. Decision-makers must weigh technical capability, regulatory experience, and operational agility when aligning with an outsourced partner, given the downstream impact on timelines, product quality, and patient safety.
How technological convergence, digital process control, and shifting client outsourcing models are reshaping competitive differentiation in small molecule CDMO services
The landscape for small molecule CDMO services is redefining value through technological convergence, regulatory expectations, and client operating models that prioritize flexibility and speed to clinic. Artificial intelligence and machine learning are increasingly applied to reaction prediction, route selection, and analytical method development, enabling teams to compress cycle times and reduce experimental iterations. Concurrently, modular and continuous manufacturing technologies are moving from pilot demonstrations into validated commercial applications, offering pathways to improve efficiency and reduce footprint while maintaining high-quality outputs.Regulatory frameworks are also adapting to these technological shifts, with agencies signaling support for advanced manufacturing approaches and data-centric submissions that emphasize real-time quality assurance. This creates opportunities for CDMOs that invest in digital process controls, quality-by-design practices, and robust data integrity systems. Meanwhile, client strategies are shifting toward flexible outsourcing models that blend strategic partnerships with tactical engagements, enabling large pharmaceutical companies and emerging biotechs alike to access capabilities on a need-basis without compromising control over critical intellectual property. These transformative shifts are collectively reshaping competitive differentiation, where technical depth, digital maturity, and a demonstrated regulatory track record become key determinants of long-term client relationships.
Assessing the operational and procurement repercussions of 2025 tariff implementations on cross-border sourcing, supplier diversification, and manufacturing resilience
The implementation of tariffs in 2025 introduces a complex layer of commercial and operational implications for supply chains that are already navigating geopolitical fragmentation and resilience planning. Tariff measures affect raw material pricing, import-export dynamics, and the comparative economics of regional manufacturing options. For organizations that rely on cross-border procurement of catalysts, reagents, specialized solvents, and advanced intermediates, tariffs can alter supplier selection criteria and incentivize nearshoring or regional diversification to mitigate cost volatility and customs-related lead times.In response, many industry participants are revising procurement strategies to emphasize supplier qualification across multiple geographies, building inventory buffers for critical reagents, and accelerating qualification of alternative suppliers to reduce exposure to tariff-driven disruptions. CDMOs that proactively demonstrate multi-regional sourcing capabilities, transparent cost pass-through practices, and flexible manufacturing footprints will provide clients with greater assurance of supply continuity. Furthermore, companies are investing in scenario planning and integrated cost modeling to quantify tariff impacts on development timelines and product lifecycles, enabling more informed negotiation and contracting approaches that preserve project momentum despite fluctuating trade barriers.
Integrating service type, development stage, therapeutic focus, customer profile, and operational scale to refine CDMO service models and client engagement strategies
Segment-level dynamics reveal differentiated demand drivers that necessitate finely tailored service offerings and commercial models. Within service type, clients require robust analytical services with rapid method development and stability testing alongside API manufacturing capabilities that span from pilot demonstrations to validated commercial operations. Formulation and packaging needs vary from early-stage flexible formats for clinical supplies to high-throughput commercial packaging lines, while process development must deliver both route screening across catalytic, reagent, and solvent variables and subsequent process optimization, route optimization, and technology transfer that ensures scale-up reliability.Development stage considerations drive expectations for agility versus robustness: preclinical and Phase I programs prize speed and exploratory flexibility, Phase II and Phase III emphasize scale-up readiness and regulatory documentation, and commercial programs demand repeatable manufacturing controls and supply chain transparency. Therapeutic area influences technical complexity and lifecycle management; oncology and CNS programs may require specialized impurity control and delivery formats, whereas infectious disease projects often prioritize speed and manufacturing scalability. Customer type shapes contractual dynamics and service portfolios, with large pharmaceutical clients seeking long-term strategic partnerships, mid-sized biopharma favoring flexible multi-project frameworks, and small biotech companies requiring focused, milestone-driven engagements. Scale considerations-from micro scale for discovery and early clinical work to pilot and commercial scale-affect facility allocation, quality systems, and investment in single-use versus stainless-steel equipment. Integrating these segmentation lenses enables service providers and clients to align technical capabilities with program-specific risk profiles and timelines.
Comparing regional strengths and strategic considerations across the Americas, Europe Middle East & Africa, and Asia-Pacific to inform CDMO location and sourcing decisions
Regional imperatives in the small molecule CDMO space are shaped by differing regulatory regimes, talent pools, and infrastructure investments. The Americas continues to command significant innovative activity supported by deep clinical ecosystems and established manufacturing clusters that prioritize high-complexity projects and regulatory experience. This region benefits from proximity to major sponsors, accessible capital markets, and a robust supplier base for advanced reagents and analytical instruments, making it a preferred location for late-stage development and commercial manufacturing where regulatory familiarity and supply chain reliability are critical.Europe, Middle East & Africa exhibits a heterogeneous landscape in which legacy chemical manufacturing strengths coexist with rapidly modernizing facilities and growing investments in advanced analytics and continuous manufacturing. Regulatory harmonization across key European markets facilitates cross-border operations, and specialized contract manufacturers serve both regional and global pipelines. Talent specialization in chemistry, process engineering, and regulatory affairs supports complex route optimization needs. Asia-Pacific presents a spectrum from high-volume, cost-competitive production hubs to centers of technical excellence that are increasingly focusing on higher-value services such as complex API synthesis, formulation innovation, and integrated development programs. Regional considerations influence decisions around nearshoring, supplier qualification, and risk allocation, with clients balancing cost, speed, and regulatory alignment when selecting geography for each program phase.
How scientific depth, digital maturity, and strategic expansion initiatives determine competitive positioning among leading small molecule CDMO service providers
Key competitors in the small molecule CDMO arena differentiate through combinations of scientific specialization, geographic footprint, digital capabilities, and regulatory track record. Some providers emphasize deep synthetic chemistry expertise and process development teams capable of managing challenging impurity profiles and stereochemical complexities, while others focus on scalable commercial manufacturing, multi-product facilities, and robust quality systems that meet stringent global regulatory expectations. A number of firms are investing heavily in advanced analytics, in-line monitoring, and digital process control to improve process understanding and reduce batch variability.Strategic partnerships, capacity expansion, and targeted acquisitions remain common routes for companies seeking to broaden service portfolios and enter new therapeutic or geographic segments. Clients increasingly evaluate providers based on demonstrated success in technology transfer, the ability to manage multi-step syntheses, and the clarity of regulatory documentation practices. Providers that can showcase case studies of accelerated route screening, efficient process optimization, and seamless transition from pilot to commercial scale tend to secure more complex, higher-value engagements. Ultimately, differentiation arises from the ability to combine technical excellence with operational reliability and transparent client collaboration models.
Actionable strategic investments and operational practices that leaders must adopt to enhance technical capability, supply resilience, and client-centric service delivery
Industry leaders should prioritize investments that balance near-term client needs with longer-term resilience and differentiation. First, enhance capabilities in process development by investing in high-throughput route screening that addresses catalytic, reagent, and solvent variables, and pair these capabilities with robust analytical development to accelerate decision-making while ensuring impurity control. Second, adopt digital tools for process modeling, real-time quality monitoring, and data integration across development and manufacturing to shorten development cycles and increase reproducibility. These investments will reduce operational risk and support regulatory submissions grounded in strong data integrity.Third, design flexible commercial models that reflect the diversity of customer types and development stages, offering modular service packages that can scale from micro and pilot operations to full commercial production. Combine this with proactive supply chain strategies that include multi-regional sourcing, strategic buffer inventories, and validated alternative suppliers to mitigate tariff and logistics risks. Fourth, cultivate cross-functional teams with expertise in therapeutic-specific requirements-such as oncology impurity management or CNS delivery challenges-to better align technical solutions with clinical objectives. Finally, pursue targeted strategic partnerships or acquisitions that close capability gaps and enable faster entry into priority regional markets, ensuring that operational investments translate into measurable client outcomes.
A transparent multi‑method research approach combining practitioner interviews, technical literature review, and thematic synthesis to validate actionable industry insights
This research synthesizes primary qualitative interviews with industry executives, process scientists, and regulatory experts alongside secondary analysis of publicly available technical literature, regulatory guidance documents, and facility announcements. Primary research focused on eliciting practitioner perspectives on process development workflows, route screening practices, analytical method priorities, and evolving client engagement models. Interview subjects were selected to represent a range of customer types including large pharmaceutical companies, mid-sized biopharma players, and smaller biotech firms, as well as CDMO service providers with capabilities spanning analytical services, API manufacturing, formulation and packaging, and multi-tiered process development.Secondary analysis examined published regulatory guidance, patent filing trends, and technology adoption indicators that highlight shifts toward modular manufacturing and digital process controls. The methodology employed triangulation to validate findings across multiple sources, and thematic coding was applied to interview transcripts to surface common pain points and opportunity areas. Care was taken to ensure methodological rigor through cross-validation of qualitative insights with observable industry activity, allowing the research to present practical implications for procurement, process optimization, and strategic partnerships without relying on proprietary commercial estimations.
Concluding perspective on how integrated scientific capability, resilient operations, and client-centric models determine sustained success in small molecule CDMO engagements
Small molecule CDMO services occupy a pivotal role in translating chemical innovation into patient-ready therapies, and success in this space depends on aligning scientific capabilities with resilient operations and client-focused commercial models. Providers that marry high-throughput route screening and rigorous process optimization with digital quality frameworks will be best positioned to serve diverse therapeutic programs and development stages. The interplay between regulatory expectations, technological adoption, and shifting procurement strategies means that flexibility and demonstrable technical depth will be the key differentiators going forward.Organizations should interpret these conclusions as a call to integrate investments across process development, analytical excellence, and supply chain diversification. By doing so, they can reduce program risk, accelerate timelines when speed is essential, and provide clients with the operational certainty required for late-stage and commercial manufacturing. Strategic clarity, coupled with operational execution, will enable CDMOs and their clients to navigate tariff-related disruptions and regional complexity while maintaining focus on delivering high-quality small molecule therapeutics to patients.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- Aenova Holding GmbH
- Akums Drugs & Pharmaceuticals Ltd.
- Almac Group Ltd.
- Apeloa Pharmaceutical Co., Ltd.
- Aspen Pharmacare Holdings Ltd.
- Bachem Holding AG
- Cambrex Corporation
- Catalent, Inc.
- CordenPharma International GmbH
- Curia Global, Inc.
- Divi’s Laboratories Ltd.
- EuroAPI
- Evonik Industries AG
- Fujifilm Diosynth Biotechnologies
- Hovione Ltd.
- Lonza Group AG
- PCI Pharma Services
- Piramal Pharma Solutions
- Porton Pharma Solutions Ltd.
- Recipharm AB
- Siegfried Holding AG
- Simtra BioPharma Solutions
- Thermo Fisher Scientific Inc.
- WuXi AppTec Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
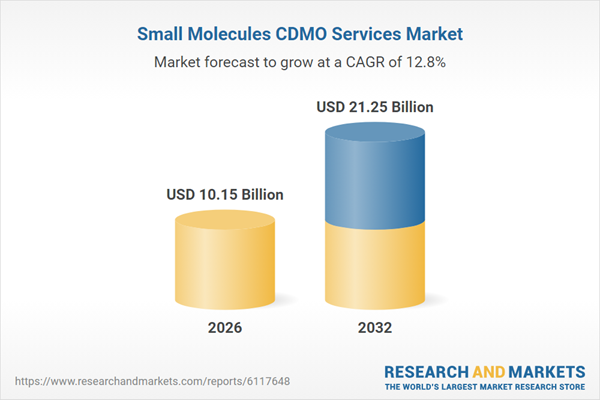
| Estimated Market Value ( USD | $ 10.15 Billion |
| Forecasted Market Value ( USD | $ 21.25 Billion |
| Compound Annual Growth Rate | 12.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |