Speak directly to the analyst to clarify any post sales queries you may have.
Comprehensive orientation to the scientific rationale and operational implications of fluorinated drug development shaping strategic R&D decisions
Fluorinated compounds have become central to modern drug discovery and development, driven by their capacity to modulate physicochemical properties, metabolic stability, and target engagement. As industry participants increasingly integrate fluorine chemistry into lead optimization strategies, the scientific and commercial landscapes have evolved in parallel, with advances spanning medicinal chemistry, formulation science, and analytical characterization. This introduction frames the essential scientific rationale behind fluorination, its practical implications for candidate selection, and the operational considerations that influence development timelines.Beyond chemistry, fluorinated therapeutics intersect with broader themes such as biologics engineering, peptide stabilization, and small molecule optimization. This convergence demands cross-functional collaboration among discovery chemists, process development scientists, regulatory specialists, and commercial strategists. Consequently, organizations pursuing fluorinated candidates must balance innovation in compound design with rigorous assessment of manufacturability, regulatory pathways, and patient-centric delivery approaches.
The remainder of this executive summary situates these technical drivers within a changing external environment. It draws links between R&D trends, policy shifts, and supply chain vulnerabilities to help informed stakeholders anticipate critical inflection points and prioritize actions that preserve competitive advantage while accelerating safe, efficacious therapies to patients.
Converging scientific innovations and operational forces are redefining how fluorinated therapeutics are designed, tested, regulated, and commercialized
The landscape for fluorinated therapeutics is being reshaped by multiple transformative shifts that collectively redefine how programs move from discovery to clinic. First, advances in synthetic methods and enabling technologies have broadened the palette of fluorinated motifs that can be incorporated efficiently and reproducibly, reducing historical barriers tied to complex chemistries. Second, improvements in analytical tools and in silico modeling now permit more precise prediction of how fluorination alters pharmacokinetics and target engagement, leading to higher-confidence candidate selection.Concurrently, therapeutic trends such as the rise of peptide therapeutics and complex biologics have created new opportunities to apply fluorination strategies beyond traditional small molecules, extending benefits like enhanced membrane permeability and proteolytic resistance. Regulatory expectations are also evolving, with authorities demanding richer characterization of novel moieties and more comprehensive safety packages, which in turn has stimulated collaboration between regulatory affairs and early development teams.
Finally, commercial and supply chain dynamics-ranging from demand for differentiated molecules to pressures on raw material sourcing-have elevated strategic procurement and supplier qualification as core competencies. Taken together, these shifts compel organizations to adopt integrated development pathways that synchronize medicinal chemistry design with manufacturability, regulatory readiness, and downstream commercialization planning.
Tariff-driven procurement complexities and supply chain reconfiguration are reshaping sourcing, manufacturing localization, and financial planning for fluorinated drug programs
Recent tariff policy changes in the United States have introduced material complications for cross-border procurement, manufacturing planning, and cost structures that underpin pharmaceutical development. For organizations reliant on global supply chains for fluorinated intermediates, reagents, or contract manufacturing, tariff impositions have prompted reassessments of sourcing strategies and supplier diversification plans. In response, procurement teams have elevated supplier due diligence and logistical contingency planning to the forefront of program risk management.These trade adjustments have also accelerated conversations about regionalization of production capacity, with firms exploring closer-to-market manufacturing nodes to mitigate exposure to tariff-driven cost variability. In parallel, finance and commercial teams are recalibrating pricing models and customer engagement strategies to preserve margin and access while maintaining competitive positioning.
From a development perspective, the tariff-related environment has reinforced the importance of securing flexible supply arrangements and advancing early-stage process robustness so that manufacturing transitions can occur with minimal disruption. Additionally, companies are investing in supply chain transparency and traceability measures to navigate compliance requirements more efficiently and to sustain continuity of clinical supplies during cross-border policy shifts.
In-depth segmentation analysis revealing how drug type, therapeutic domain, administration route, development stage, compound class, end user context, and distribution channels interact to determine development priorities
Segmentation provides a structured lens through which to evaluate where fluorinated strategies deliver greatest clinical and commercial traction. Based on drug type, the landscape spans biologics, peptides, and small molecules; within biologics, monoclonal antibodies, nucleic acids, and recombinant proteins each present distinct opportunities for targeted fluorination to enhance stability, delivery, or analytical traceability. Based on therapeutic area, relevant applications include treatments for autoimmune disorders, cardiovascular conditions, central nervous system indications, oncology, and respiratory diseases; cardiovascular subdomains such as atherosclerosis, heart failure, and hypertension, oncology subdomains encompassing hematological and solid tumor approaches, and respiratory subdomains including asthma and chronic obstructive pulmonary disease each drive specific design considerations for fluorinated compounds.Based on route of administration, inhalation, injectable, oral, topical, and transdermal modalities impose divergent formulation and safety constraints that influence candidate selection and development pathways. Based on end user, clinics, home care, hospitals, and retail pharmacies require different stability, packaging, and delivery solutions to ensure real-world usability. Based on development stage, programs range from preclinical and early phase investigations through Phase I, Phase II, Phase III, and approved products, with each stage carrying distinct evidence generation and regulatory documentation needs. Based on compound class, fluorinated aliphatics, fluorinated amino acids, fluorinated heterocycles, and fluoroarenes offer discrete properties that medicinal chemists leverage to tune potency, selectivity, and metabolic profile. Based on distribution channel, direct sales, distributors, and e-commerce channels shape commercialization planning, reimbursement dialogues, and post-market surveillance approaches.
Synthesizing across these segmentation dimensions reveals where technical leverage from fluorination intersects with clinical need and commercial access. For example, inhaled and injectable modalities frequently prioritize molecular stability and controlled release, making fluorinated heterocycles and fluorinated amino acids especially relevant for respiratory and peptide-based therapeutics. Similarly, oncology and CNS programs often demand precise target engagement and blood-brain barrier considerations where small molecules with specific fluorinated motifs can provide competitive differentiation. Understanding these layered segmentation dynamics enables strategic allocation of resources and more targeted translational research efforts.
Comparative regional dynamics and infrastructure distinctions that influence development sequencing, manufacturing localization, and regulatory strategies across global regions
Regional dynamics are instrumental in shaping strategic choices for fluorinated therapeutics, as regulatory frameworks, manufacturing capacity, and clinical trial infrastructure differ across geographies. In the Americas, strong clinical research ecosystems and advanced manufacturing networks support rapid iteration and early human studies, yet supply chain dependencies and import/export policies can influence sourcing decisions. In Europe, Middle East & Africa, regulatory harmonization efforts across several jurisdictions coexist with diverse reimbursement landscapes, requiring tailored regulatory strategies and localized evidence generation to secure access. In Asia-Pacific, expanding biotech hubs, growing contract development and manufacturing capabilities, and increasing domestic investment in drug development create fertile ground for scale-up and regional partnerships, though IP management and regulatory timelines vary significantly by country.Geographic considerations also affect patient recruitment strategies, prevalence of therapeutic indications, and the availability of specialized clinical sites for complex interventions. Moreover, regional differences in raw material sourcing, reagent availability, and vendor ecosystems shape manufacturing risk profiles and supplier selection. Taken together, these regional insights inform decisions about where to place pilot manufacturing, how to sequence regulatory filings, and which geographies to prioritize for early commercialization efforts, enabling organizations to align operational design with strategic expansion plans.
Organizational capability profiles and partnership models that distinguish successful developers, contract manufacturers, and specialty suppliers in advancing fluorinated therapeutics
Leading organizations advancing fluorinated therapeutics are adopting multidimensional approaches that combine deep chemistry expertise with capabilities in process development, regulatory science, and strategic partnerships. Innovator pharmaceutical companies are increasingly embedding fluorination expertise within discovery teams and are forging alliances with specialized contract development and manufacturing organizations to accelerate translation from bench to clinic. Contract research organizations and CDMOs with demonstrated capacity for handling fluorinated reagents and conducting specialized analytical assays have become critical enablers, offering scale-up experience and quality systems that support complex submissions.Specialty active pharmaceutical ingredient manufacturers and fine-chemical suppliers that invest in secure sourcing and scalable synthesis routes are differentiating themselves by offering robust supply agreements and regulatory documentation packages. Similarly, companies that couple medicinal chemistry with advanced formulation science to address route-of-administration challenges-such as inhalation or transdermal delivery-are better positioned to de-risk clinical programs. Finally, organizations that proactively integrate regulatory intelligence, toxicology expertise, and pharmacokinetic modeling into early development cycles tend to streamline approval pathways and reduce late-stage surprises, enabling more predictable program progression.
Practical and prioritized actions for R&D, supply chain, regulatory, and commercial teams to accelerate development and reduce risk for fluorinated drug programs
Industry leaders should prioritize integrated program design that aligns medicinal chemistry objectives with downstream manufacturing and regulatory constraints to reduce development friction. Investing early in process-development expertise and supplier qualification for fluorinated intermediates will pay dividends by minimizing scale-up delays and preserving clinical supply continuity. Additionally, embedding regulatory strategy and toxicology planning into discovery stages mitigates the risk of late-stage data gaps and clarifies evidence requirements for novel fluorinated motifs.Leaders should also diversify manufacturing footprints and cultivate strategic partnerships across geographies to enhance resilience against trade policy shifts and supply disruptions. Where appropriate, establishing regional pilot manufacturing capabilities or long-term agreements with capable contract manufacturers can reduce exposure to cross-border cost variability and logistical constraints. On the commercialization side, aligning formulation development with end-user needs-whether for home care, hospital administration, or retail distribution-will improve adherence outcomes and patient experience. Finally, organizations should leverage advanced analytics and modeling to predict how fluorination will affect ADME profiles and to inform candidate prioritization, thereby focusing resources on programs with the clearest clinical rationale and feasible development pathways.
Robust mixed-methods approach integrating expert interviews, literature synthesis, regulatory scanning, and evidence triangulation to produce validated, actionable insights
The research methodology underpinning these insights combined a structured synthesis of contemporary scientific literature, targeted primary interviews with subject-matter experts across discovery, development, regulatory, and manufacturing domains, and a systematic scan of public regulatory guidance and policy announcements. Evidence triangulation was applied to reconcile divergent perspectives and to identify recurring themes across independent data streams, ensuring robustness of conclusions drawn about technical feasibility and strategic implications.Analytical approaches included qualitative coding of interview transcripts to surface dominant risk factors and strategic priorities, comparative analysis of regulatory pathways relevant to novel chemical entities, and scenario mapping to evaluate the operational consequences of supply chain disruptions and tariff policy changes. Case-level examination of representative fluorinated programs provided practical examples of successful integration of chemistry innovation with process development and regulatory planning. Throughout, emphasis was placed on transparent assumptions, explicit documentation of uncertainty, and consultative validation with external experts to ensure the findings are actionable for decision-makers.
Strategic synthesis of technical, regulatory, and operational imperatives to guide successful advancement of fluorinated drug programs toward clinical and commercial readiness
In conclusion, fluorinated therapeutics represent a strategically important domain where chemistry-driven innovation can materially enhance drug performance across diverse modalities and therapeutic areas. The evolving interplay between synthetic capability, regulatory expectations, and commercial dynamics requires organizations to adopt integrated development strategies that account for compound class traits, route-specific formulation needs, and geographic supply chain considerations. Proactive investment in process development, supplier qualification, and regulatory planning reduces program risk and enables more predictable progression through clinical milestones.Moreover, the current policy environment and supply chain pressures underscore the importance of operational resilience and strategic sourcing. Organizations that prioritize cross-functional collaboration, cultivate specialized partnerships, and apply advanced modeling to candidate selection will be best positioned to translate fluorination advantages into safe, effective, and accessible therapies. These conclusions offer a strategic compass for stakeholders seeking to navigate the technical and commercial complexities inherent to fluorinated drug development.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
19. China Fluorinated Drugs Market
Companies Mentioned
- AbbVie Inc.
- Amgen Inc.
- AstraZeneca PLC
- Cipla Limited
- Eli Lilly and Company
- F. Hoffmann-La Roche AG
- GSK plc
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
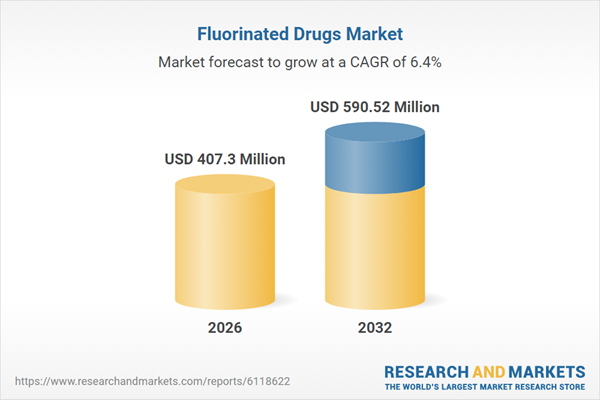
| Estimated Market Value ( USD | $ 407.3 Million |
| Forecasted Market Value ( USD | $ 590.52 Million |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |