Speak directly to the analyst to clarify any post sales queries you may have.
A practical orientation to the evolving microbial testing ecosystem highlighting technology, regulation, and operational choices that drive laboratory resilience
The microbial testing services landscape is at a strategic inflection point driven by accelerating regulatory scrutiny, evolving pathogen threats, and rapid technological innovation. This executive summary synthesizes the market dynamics that matter to laboratory directors, procurement leads, technology vendors, and policy-makers. It focuses on practical implications rather than projections, highlighting the operational levers that stakeholders can use to strengthen quality assurance, reduce time-to-result, and improve risk management across end uses ranging from food safety to pharmaceutical sterility assurance.Emerging molecular platforms, a surge in demand for rapid and on-site testing, and persistent emphasis on supply chain resilience are reshaping how testing is procured and delivered. At the same time, fragmentation across service models-contract testing, in-house laboratories, and hybrid approaches-creates both complexity and opportunity. This introduction frames the subsequent analysis by clarifying the drivers of change, the interplay between technology and regulation, and the practical choices organizations face when balancing speed, sensitivity, and cost. The tone throughout is pragmatic: focus on actionable insight, operational readiness, and the strategic alignment of testing capabilities with organizational risk tolerance and compliance obligations.
How converging technological advances, heightened regulatory expectations, and shifting end-user demands are reshaping microbial testing service delivery
Transformative shifts in microbial testing are being driven by three overlapping vectors: technological maturation, regulatory evolution, and changing end-user expectations. Advances in next-generation sequencing and digital PCR have improved sensitivity and expanded the range of detectable targets, while miniaturized biosensors and lateral flow rapid tests have shortened time-to-answer and enabled decentralized testing in field and on-site contexts. These technology trends are accompanied by growing demand for interoperability between laboratory information management systems and cloud-enabled analytics, which in turn increases the value of standardized data outputs and structured result reporting.Regulatory agencies globally are raising the bar for validation and traceability, prompting laboratories to adopt more rigorous quality management systems and to document chain-of-custody and method validation more comprehensively. Meanwhile, end users in food and beverage, pharmaceuticals, water utilities, and clinical settings increasingly prioritize risk-based testing strategies and faster actionable results. Consequently, service providers are innovating not only in assay chemistry and instrumentation but also in service delivery models, offering bundled analytics, remote monitoring, and consulting to help clients translate results into operational decisions. Because these trends intersect, stakeholders that align procurement, technical validation, and regulatory planning will be best positioned to capture value as the landscape continues to evolve.
Anticipating supply chain reconfiguration and validation burdens as tariff dynamics prompt procurement diversification and localized sourcing efforts
The cumulative effects of tariff policy and trade frictions originating in the United States as of 2025 have introduced new considerations for supply chain design and supplier selection across the microbial testing ecosystem. Tariff-related cost pressures have made the sourcing of critical consumables, specialized reagents, and precision components more complex. Laboratories that previously relied on single-source imports for items such as chromatography columns, sequencing flow cells, PCR reagents, and cartridge-based consumables now face the need to diversify suppliers, build buffer inventories, or localize procurement where feasible. This dynamic increases lead times and elevates the importance of procurement agility and supplier risk assessment.Beyond direct cost implications, tariff-driven re-shoring and near-shoring initiatives have implications for qualification timelines and regulatory documentation. When suppliers change due to tariff economics, method re-validation and supplier audits become more frequent, requiring additional internal resources and planning. In some cases, equipment OEMs have adjusted distribution strategies to mitigate tariff exposure, accelerating partnerships with regional distributors and contract manufacturing organizations. For organizations that prioritize continuity, early engagement with vendors to understand supply chain footprints and tariff mitigation strategies will reduce operational disruption. Finally, while tariffs alter unit economics, they also catalyze investment in consumable-sparing technologies, assay multiplexing, and automation that reduce per-test dependency on imported reagents.
Granular segmentation analysis connecting end uses, test modalities, technologies, sample matrices, and service delivery models to practical decision criteria
Insightful segmentation illuminates where demand pressure, technological adoption, and service model preferences intersect across microbial testing end uses, test types, technologies, sample types, and service models. Based on end use, testing requirements diverge among agriculture, clinical diagnostics, environmental monitoring, food and beverage safety, pharmaceutical and biopharmaceutical quality assurance, and water testing, with the food and beverage category exhibiting distinct needs across beverages, dairy, meat and poultry, and seafood. Pharmaceutical and biopharmaceutical testing demands further granularity across active pharmaceutical ingredient testing, biologics analysis, and finished drug formulation testing, while water testing priorities differ among drinking water, recreational water, and wastewater monitoring. These end-use distinctions shape assay sensitivity expectations, turnaround time tolerances, and documentation rigor.Based on test type, methodological choices range from culture-based methods and enumeration techniques to immunoassays, molecular tests, and rapid diagnostics. Enumeration approaches such as flow cytometry and microscopy serve quantitative needs, while immunoassays-Elisa and lateral flow formats-provide targeted antigen or antibody detection. Molecular workflows include polymerase chain reaction and next-generation sequencing, with NGS enabling broader surveillance and PCR offering rapid, target-specific detection. Rapid tests that leverage biosensor platforms and lateral flow rapid formats support point-of-need use cases where speed is paramount.
Based on technology, the market deploys chromatography, Elisa platforms, flow cytometry, next-generation sequencing, and PCR systems. Chromatography applications utilize both gas chromatography and HPLC for chemical and contaminant profiling, whereas next-generation sequencing workflows may rely on platforms such as short-read and long-read technologies. PCR methods continue to evolve with digital PCR and quantitative PCR providing complementary sensitivity and quantitation capabilities. Based on sample type, laboratories manage diverse matrices including air, food, soil, swabs, and water, with food subtypes spanning dairy, meat and poultry, and produce, and swab protocols differentiating equipment swabs from surface swabs. Based on service model, organizations choose between contract testing and in-house testing, where contract testing options include ISO-accredited laboratories and third-party contract research organizations, while in-house models may feature on-site laboratories and hybrid arrangements that blend external and internal capabilities.
Taken together, these segmentation layers reveal that optimal testing choices are contingent on the intersection of regulatory requirements, operational tempo, and cost constraints. Organizations deploying complex biologics assays will prioritize advanced molecular and chromatographic capabilities alongside robust documentation, whereas food processors often balance rapid screening with periodic confirmatory culture-based testing. Meanwhile, municipal water utilities tend to emphasize standardized methods and regulatory compliance, favoring stable vendor relationships and validated enumeration techniques. Understanding segmentation at this level enables procurement teams to match technology investments and service agreements to specific operational and compliance objectives.
Regional differentiation in regulatory focus, technology adoption, and supply chain strategy across the Americas, Europe Middle East & Africa, and Asia-Pacific
Regional forces shape demand patterns, technology adoption, and regulatory priorities across the Americas, Europe, Middle East & Africa, and Asia-Pacific, producing differentiated operational strategies for microbial testing stakeholders. In the Americas, laboratories and service providers often emphasize rapid adoption of next-generation molecular tools and integrated informatics to support clinical diagnostics, food safety, and environmental monitoring, with a strong focus on scalability and vendor partnerships that offer turnkey solutions. Regulatory frameworks tend to emphasize validation documentation and traceability, prompting investment in laboratory information management systems and quality assurance processes.In Europe, Middle East & Africa, regulatory harmonization efforts and robust food safety standards drive conservative validation approaches, with a premium placed on accredited testing, method standardization, and supply chain transparency. Middle Eastern and African markets exhibit variable infrastructure maturity, which creates opportunities for contract testing providers and mobile testing solutions to fill capability gaps, particularly for water and environmental surveillance. Across these regions, demand for sustainability-aligned testing and reduced waste in consumables is influencing procurement decisions.
In Asia-Pacific, rapid industrialization and expanding pharmaceutical manufacturing capacity have increased demand for both advanced molecular testing and traditional culture-based methods. The region shows fast uptake of decentralized testing models and automation to serve high-volume food and beverage processing and growing clinical diagnostics markets. Local manufacturing of reagents and instrumentation has also gained momentum, driven in part by policy incentives and the desire to mitigate import dependency. Overall, regional distinctions inform where to prioritize vendor partnerships, manufacturing localization, and regulatory engagement to ensure operational continuity and compliance.
How integrated platform strategies, targeted partnerships, and supply chain investments are defining competitive advantage among testing service providers and vendors
Competitive dynamics within the microbial testing services ecosystem reflect a mix of established instrumentation suppliers, specialized contract testing laboratories, reagent manufacturers, and software and analytics providers. Market leaders are investing in platform integration, service bundling, and partnerships that extend capabilities beyond instrumentation to include data analytics, method validation support, and regulatory consulting. At the same time, niche players that specialize in rapid tests, biosensors, or specific sample matrices such as wastewater have carved defensible positions by solving high-value, time-sensitive problems for particular end users.Strategic moves among companies include vertical integration of reagent manufacturing to secure supply, alliances between instrument OEMs and contract testing labs to expedite method deployment, and acquisitions that bring complementary technologies into single portfolios. Investments in digital tools for remote monitoring, predictive maintenance, and instrument performance tracking are becoming standard operating practice as firms seek to reduce downtime and improve customer experience. For contract laboratories and third-party service providers, demonstrating ISO accreditations, robust quality systems, and transparent chain-of-custody documentation are essential competitive differentiators. Finally, partnerships with regional distributors and local manufacturing agreements are increasingly important as companies respond to supply chain risk and tariff pressures, enabling faster turnaround times and localized support for high-demand customers.
Actionable strategies to strengthen supply resilience, accelerate modular automation, and align testing models with operational priorities for immediate impact
Industry leaders should adopt a multi-pronged, pragmatic approach to secure operational resilience, accelerate innovation adoption, and optimize cost structures. First, prioritize supplier diversification and validation roadmaps that reduce single-source dependency for critical reagents, consumables, and instrument parts. By establishing pre-qualified alternative suppliers and staged re-validation protocols, organizations can shorten qualification cycles when supply disruptions occur and maintain continuity of testing services.Second, invest selectively in modular automation and assay multiplexing to reduce per-test reagent consumption and increase throughput without sacrificing sensitivity. Modular automation enables laboratories to scale incrementally and reconfigure workflows in response to changing test mixes. Third, strengthen data integration by implementing laboratory information management systems that support standardized reporting, instrument interoperability, and secure cloud-enabled analytics. Reliable data flows accelerate decision-making, support regulatory reporting, and enable advanced quality metrics tracking.
Fourth, align service models to end-use priorities by matching rapid, on-site testing options to operationally critical points while reserving high-complexity molecular or chromatographic analyses for centralized laboratories. This hybrid approach reduces turnaround time for routine decisions while preserving access to deep analytical capacity when confirmatory testing is required. Fifth, proactively engage with regulators and accreditation bodies to co-design validation plans that anticipate changes in supplier footprints and method updates, thereby reducing unplanned audit burdens. Finally, consider strategic partnerships with contract testing laboratories or localized manufacturing partners to mitigate tariff exposure and improve time-to-delivery for high-volume consumables and standardized assays.
A mixed-methods validation and supplier mapping methodology integrating practitioner interviews, technical validation, and regulatory contextualization for operational relevance
The research approach underpinning this analysis combines primary interviews, technical validation, and structured secondary synthesis to ensure findings are grounded in operational reality. Primary data was gathered through structured interviews with laboratory directors, procurement leaders, quality assurance managers, and technology vendors to capture on-the-ground perspectives about validation challenges, procurement behaviors, and evolving method preferences. These qualitative inputs were triangulated with technical validation exercises that assessed the operational characteristics of representative assay families, including molecular platforms, immunoassays, culture-based methods, chromatography, and rapid biosensor tests.Secondary synthesis drew on regulatory guidance documents, standards for laboratory accreditation, white papers from instrumentation vendors, and publicly available policy analysis to contextualize operational insights. Supplier mapping and capability matrices were developed to evaluate typical service model configurations, including ISO-accredited contract laboratories, third-party CROs, and on-site laboratory deployments. Special emphasis was placed on traceability and method transfer considerations when suppliers or platforms change, and sensitivity analyses focused on the practical implications of procurement disruption due to trade policy changes. Throughout, a conservative, evidence-based approach prioritized verifiable operational impacts and recommended mitigation actions that laboratories can implement within existing quality and regulatory frameworks.
Synthesis of operational imperatives showing how procurement agility, modular technology, and robust data governance combine to enhance laboratory resilience
In conclusion, the microbial testing services environment is being reshaped by convergent forces that reward operational flexibility, supplier transparency, and targeted technology adoption. Organizations that proactively redesign procurement strategies, invest in modular automation and data interoperability, and align service delivery models to the specific demands of end-use segments will reduce risk and accelerate time-to-action. Tariff dynamics and supply chain realignments underscore the importance of supplier diversification and method re-validation planning, while regional differences in regulatory posture and local manufacturing capacity should inform vendor selection and deployment strategies.Ultimately, the most resilient organizations will blend centralized analytical depth with decentralized rapid testing, supported by robust data systems and clear governance around quality and documentation. The practical recommendations in this summary are designed to be implementable within typical laboratory governance cycles and to reduce the friction associated with supplier changes, method transfers, and regulatory review. Stakeholders that operationalize these insights will be better positioned to maintain compliance, sustain throughput, and respond rapidly to emerging contamination events or supply chain shocks.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Microbial Testing Service Market
Companies Mentioned
The key companies profiled in this Microbial Testing Service market report include:- ALS Limited
- bioMérieux
- Bureau Veritas SA
- Charles River Laboratories International, Inc.
- Eurofins Scientific SE
- Intertek Group plc
- Laboratory Corporation of America Holdings
- Mérieux NutriSciences
- Neogen Corporation
- SGS SA
- STERIS plc
- TÜV SÜD AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 190 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
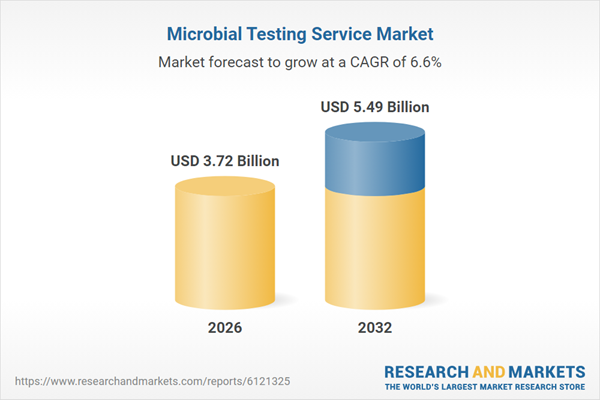
| Estimated Market Value ( USD | $ 3.72 Billion |
| Forecasted Market Value ( USD | $ 5.49 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |