Speak directly to the analyst to clarify any post sales queries you may have.
Aquaculture Immunostimulants Become Core to Modern Health Management as Producers Pursue Resilience, Compliance, and Predictable Farm Outcomes
Aquaculture is operating in an environment where disease pressure, regulatory scrutiny, and cost volatility converge at the farm gate. Producers are expected to deliver consistent yields while reducing antibiotic dependence, meeting retailer standards, and navigating biosecurity risks that can cascade across a region in weeks. In this context, aquaculture immunostimulants have moved from a niche “supportive” input to a central component of health management programs that prioritize robustness, survivability, and predictable performance.Immunostimulants are increasingly positioned as tools that prime innate immunity, improve stress tolerance, and enhance responses to vaccination and husbandry interventions. Their role becomes most visible during predictable stress windows such as transfer, grading, seasonal temperature shifts, and salinity changes, as well as during outbreaks where producers need non-antibiotic options that align with residue expectations. At the same time, the category is becoming more technical, with clearer differentiation between product types, delivery formats, and species-specific application protocols.
What makes the current moment decisive is that adoption is no longer driven only by “does it work,” but also by “can it be documented.” Buyers are asking for traceability, repeatable outcomes, and compatibility with certification frameworks. Consequently, the market conversation is shifting toward standardized trial design, farm-level data capture, and integrated health plans that combine nutrition, vaccination, genetics, and environmental management. This executive summary frames the strategic forces reshaping the landscape, the implications of evolving tariffs and trade dynamics, the most meaningful segmentation patterns, and the competitive and regional cues that matter for decision-makers.
From Reactive Treatments to Proactive Resilience: How Evidence, Functional Nutrition, and Compliance Pressures Are Redefining Immunostimulants
The most transformative shift is the transition from reactive treatment to proactive resilience. Historically, many operations responded to disease with therapeutics after mortality signals emerged. Today, producers and integrators are designing year-round health calendars, where immunostimulants are deployed as preventive inputs around high-risk events. This approach reflects hard lessons from recurring pathogen cycles and the economic reality that early mortality and growth setbacks cannot be recovered easily within fixed production windows.At the product level, the landscape is moving toward evidence-backed differentiation. Stakeholders increasingly distinguish between beta-glucans and other yeast-derived fractions, plant-based phytogenics, bacterial derivatives, and complex blends that target multiple immune pathways. As a result, suppliers are investing in mode-of-action narratives supported by farm trials that measure not only survival but also feed conversion, growth consistency, and robustness under stress. In parallel, microencapsulation and stabilization technologies are becoming more important as farms demand performance through pelleting, varying water temperatures, and extended storage.
Another major shift is the integration of immunostimulants into functional nutrition and precision feeding. Producers are optimizing inclusion rates by life stage and by risk profile rather than applying uniform dosing. Hatcheries and nurseries are expanding early-life immune programming concepts, aiming to set a stronger baseline before animals face the pathogen-rich grow-out environment. For finfish and shrimp alike, the combination of feed-based immunostimulants with water-applied solutions is evolving into hybrid protocols that reflect operational realities and disease epidemiology.
Finally, sustainability and compliance pressures are pushing the category toward greater transparency. Retailers and regulators increasingly scrutinize antibiotic usage, and many buyers prefer health tools that support antibiotic stewardship without compromising welfare. This has accelerated demand for products with consistent raw material sourcing, clear labeling, and documentation that can be incorporated into audit trails. Taken together, these shifts are elevating immunostimulants from discretionary additives to strategic inputs that shape profitability, market access, and risk management.
United States Tariffs in 2025 Reshape Aquaculture Immunostimulant Supply Chains Through Cost Pass-Through, Origin Scrutiny, and Reformulation Incentives
The cumulative impact of United States tariffs in 2025 is best understood through supply-chain friction, cost pass-through behavior, and procurement redesign. Aquaculture immunostimulants often rely on globally sourced inputs, including yeast fractions, botanical extracts, fermentation-derived components, and specialty excipients. When tariff schedules increase costs on certain imported intermediates or finished goods, manufacturers face immediate decisions: absorb margin pressure, reformulate to alternative inputs, relocate processing steps, or renegotiate supplier contracts.One of the most tangible outcomes is a renewed emphasis on origin transparency and documentation. Importers and formulators are investing more heavily in classification diligence, product documentation, and broker coordination to reduce the risk of unexpected duties and border delays. Even when tariffs apply to a subset of components, the ripple effect can alter lead times and inventory strategies across entire product lines, particularly for premixes and branded finished feed additives.
For buyers, 2025 tariff dynamics can translate into tighter supplier qualification standards and more multi-sourcing. Large integrators and feed mills are increasingly reluctant to depend on single-origin inputs for critical health products. This pushes suppliers to demonstrate continuity plans, regional stocking strategies, and validated alternative formulations that preserve efficacy. In practice, competitive advantage often accrues to companies that can offer dependable supply and stable specifications, even if unit costs are slightly higher.
Tariffs can also influence innovation pathways. When certain ingredient classes become more expensive or uncertain, formulators may pivot toward domestically available substrates, local fermentation capacity, or plant-based alternatives with less exposure to trade risk. Over time, this can reshape the product mix sold into the U.S. and affect how global suppliers prioritize registrations, technical support staffing, and partnerships with domestic feed producers. The net effect is not merely price movement; it is a reconfiguration of how value is communicated, how supply risk is priced, and how quickly new products can scale under a more complex trade environment.
Segmentation Reveals Divergent Buying Logic by Product Type, Delivery Route, Form, Species, and End User Expectations for Proof and Practicality
Segmentation patterns in aquaculture immunostimulants reveal that purchasing logic varies most sharply by how products are formulated, delivered, and justified within farm economics. In product-type terms, beta-glucans continue to be treated as a benchmark ingredient because many buyers recognize their role in innate immune activation and stress resilience. However, phytogenics and herbal extracts are gaining ground where farms prioritize multi-functional benefits such as appetite support, gut health, and antioxidant protection, especially under thermal stress. Probiotics and synbiotics often enter the immunostimulant conversation through the lens of microbiome management, with buyers seeking stable performance across variable water conditions. Meanwhile, vitamins, minerals, and amino acid-based immune support are frequently bundled into broader functional feed positioning, where the immunostimulant claim must be balanced against cost sensitivity.When viewed through the lens of application, feed additives dominate day-to-day usage because they fit standard feeding routines and can be scaled across sites with minimal labor disruption. Yet injectables and immersion approaches remain strategically important in hatchery and high-value finfish contexts, where precision dosing and early-life interventions can materially alter survival trajectories. Water treatment and pond-applied solutions have a distinct role in shrimp and extensive systems where environmental management and pathogen load reduction are treated as part of immunity support, even if the mechanism is indirect.
Form also shapes adoption. Powder formats appeal to premix manufacturers and farms that value flexibility, but they can raise handling and homogeneity challenges. Liquid formats support rapid application, top-coating, and on-farm adjustments, though stability and cold-chain requirements can become constraints. Granular and microencapsulated forms are increasingly selected for durability through pelleting and for targeted release in the gut, which matters as farms demand consistency across feed manufacturing conditions.
Species segmentation is equally decisive. Shrimp operations tend to evaluate immunostimulants based on survival under episodic disease pressure, tolerance to water-quality swings, and compatibility with biofloc or pond management practices. Salmonids often prioritize integration with vaccination programs and performance during transfer, smoltification, and temperature transitions. Carp and tilapia producers typically focus on robust, cost-effective solutions that can be applied at scale and that align with varying levels of farm sophistication.
Finally, end-user segmentation clarifies how value is captured. Large integrated producers and feed manufacturers increasingly demand documentation, repeatability, and technical service, while small and mid-sized farms may prioritize accessibility, ease of use, and visible short-term outcomes. Offline channels through distributors still matter in many regions because they provide credit and agronomic-style advice, but online and direct technical sales are growing where traceability, training content, and rapid replenishment improve the buyer experience. Across these segmentation dimensions, the most successful suppliers align product claims with operational realities, measurable outcomes, and the decision criteria of each buyer segment rather than relying on generic “immunity boosting” narratives.
Regional Adoption Patterns Reflect Species Mix, Antibiotic Stewardship, and Farm Sophistication Across the Americas, Europe, Middle East, Africa, and Asia-Pacific
Regional dynamics in aquaculture immunostimulants are shaped by species concentration, regulatory posture on antibiotics, feed manufacturing sophistication, and the maturity of health management practices. In the Americas, demand is strongly linked to industrial-scale salmonid production in the south and diversified warm-water aquaculture in the north. Buyers increasingly emphasize traceability, residue avoidance, and documentation that supports retailer and processor requirements, which favors suppliers that combine product performance with robust technical support.Across Europe, the market is characterized by stringent compliance expectations, higher scrutiny of health claims, and strong alignment with welfare and sustainability narratives. As a result, adoption often depends on clear functional positioning, high-quality manufacturing standards, and compatibility with established vaccination and biosecurity protocols. Northern European salmonid systems tend to integrate immunostimulants into structured health calendars, whereas Mediterranean and inland systems may focus on stress mitigation and seasonal risk management.
The Middle East shows a pattern of rapid capability building, where new and expanding aquaculture projects seek standardized health programs suited to warm-water environments and, in some cases, saline or recirculating systems. Here, immunostimulants are commonly evaluated for their ability to reduce production volatility under heat stress and variable water quality, with procurement often favoring suppliers that can provide training, dosing guidance, and reliable logistics.
Africa presents a dual reality: high growth potential in tilapia and catfish alongside uneven access to technical inputs and veterinary support. In many settings, immunostimulants compete with basic farm upgrades, so adoption improves when products are packaged with practical guidance, straightforward protocols, and clear on-farm value demonstration. Distribution networks and local partnerships become especially important to build trust and continuity.
Asia-Pacific remains the most complex and influential region due to the scale of shrimp and finfish production, dense farming geographies, and frequent disease challenges. In shrimp-intensive markets, immunostimulant adoption is often driven by outbreak cycles and the need for non-antibiotic tools that can be rapidly deployed. In advanced finfish systems, the focus shifts toward functional feeds, hatchery programming, and precision application. Across the region, suppliers win by tailoring formulations to local feed practices, water conditions, and regulatory expectations while maintaining consistent quality. Taken together, these regional patterns show that success requires more than exporting a product; it requires adapting evidence, service models, and supply strategies to the operational and regulatory realities of each geography.
Competitive Advantage Now Favors Immunostimulant Providers That Combine Scientific Proof, Quality Assurance, and On-Farm Technical Enablement
Company strategies in aquaculture immunostimulants increasingly converge on three competitive arenas: scientific credibility, manufacturing and supply reliability, and field-level technical enablement. Leading suppliers are strengthening their positioning through controlled trials, clearer mechanistic explanations, and application protocols tailored by species and life stage. This is a shift away from generalized claims and toward product narratives that can stand up to procurement scrutiny from integrators, feed mills, and processors.Portfolio architecture is another defining feature. Many companies are building “systems” rather than single products, pairing immunostimulants with gut health solutions, water-quality aids, or post-stress recovery formulas. This bundling aligns with how farms actually manage risk, especially during transfer events or seasonal stress peaks. It also creates a pathway for suppliers to participate earlier in the health planning cycle, influencing inclusion strategies rather than competing only on price at the point of reorder.
Operational excellence is becoming a differentiator as well. Customers increasingly ask about raw material traceability, batch-to-batch consistency, contaminant controls, and stability through feed processing. Companies that can demonstrate robust quality systems, validated supply continuity, and regional warehousing are better positioned when buyers are under pressure to avoid downtime and to maintain consistent feed performance.
Finally, the competitive field is being shaped by collaboration. Partnerships with feed manufacturers, hatcheries, and veterinary networks help suppliers embed products into standard operating procedures. Co-development projects and private-label arrangements are also expanding, particularly where feed companies want differentiated health claims while controlling formulation and supply. Across these dynamics, the most advantaged companies behave less like additive vendors and more like health-program partners, combining product innovation with training, diagnostics alignment, and measurable performance tracking.
Leaders Can Convert Immunostimulants into Predictable Performance by Standardizing Protocols, Validating Outcomes, and Building Supply Resilience
Industry leaders can strengthen outcomes by treating immunostimulants as a managed program rather than a discretionary input. Start by defining use cases tied to operational stress points such as grading, transfer, seasonal temperature swings, and known pathogen windows. From there, standardize protocols by species and life stage, including clear triggers for initiation, duration of application, and success metrics that go beyond survival to include growth uniformity and feed efficiency.Next, raise the bar on validation. Require suppliers to provide evidence that reflects your farming conditions, not only laboratory outcomes. Prioritize partners that can support on-site trials with sound controls, consistent dosing, and transparent reporting. In parallel, invest in data capture at the farm level so that performance can be evaluated across cycles, sites, and seasons, enabling procurement decisions grounded in repeatable results.
Supply resilience should be addressed proactively in light of tariff and logistics uncertainty. Qualify multiple sources for critical products, validate alternative formulations, and negotiate specifications that protect performance while allowing flexibility in ingredient sourcing. Where possible, align inventory strategies with disease-risk calendars to avoid shortages during peak need.
Leaders should also align immunostimulant adoption with antibiotic stewardship and certification goals. Position products within an integrated health framework that includes biosecurity, vaccination where applicable, optimized nutrition, water quality management, and staff training. Finally, communicate internally with clear ROI logic tied to reduced volatility and improved predictability, which helps sustain adoption even when input costs fluctuate.
A Multi-Source Methodology Combines Value-Chain Mapping, Expert Interviews, and Technical Validation to Translate Immunostimulant Trends into Decisions
The research methodology for assessing the aquaculture immunostimulant landscape integrates structured primary engagement with rigorous secondary analysis to build a practical, decision-oriented view. The process begins with mapping the value chain across ingredient suppliers, formulators, feed manufacturers, distributors, and farm operators, ensuring that insights reflect how products move from development to on-farm application. This mapping is used to define the most relevant segmentation lenses and to identify where adoption decisions are made.Primary research emphasizes expert interviews across the ecosystem, including aquaculture health managers, nutritionists, hatchery operators, feed mill decision-makers, and technical sales leaders. These conversations focus on real-world application protocols, perceived performance under stress, barriers to adoption, procurement criteria, and how regulatory and trade conditions influence purchasing behavior. To improve reliability, insights are triangulated across multiple stakeholder types and geographies rather than relying on a single viewpoint.
Secondary research consolidates publicly available technical literature, regulatory guidance, product documentation, and corporate disclosures to validate mechanisms, common claims, and quality expectations. This step also captures evolving themes such as antibiotic stewardship, functional feeds, and traceability requirements. Throughout the workflow, the analysis applies consistency checks to resolve conflicting inputs and to separate emerging signals from established practices.
Finally, the findings are synthesized into actionable frameworks that connect segmentation, regional dynamics, and competitive behavior. The goal is not only to describe the market environment, but to equip decision-makers with a clear understanding of where immunostimulants deliver the most operational value, how procurement risks are changing, and what capability investments are most likely to improve outcomes across production cycles.
Immunostimulants Shift from Optional Additives to Strategic Levers for Health Predictability, Stewardship Alignment, and Operational Risk Control
Aquaculture immunostimulants are increasingly central to how producers manage health, performance stability, and compliance expectations in an era of persistent disease risk and heightened scrutiny of antibiotic use. The category is maturing quickly, with clearer differentiation by ingredient class, delivery approach, and the kinds of evidence buyers require. At the same time, operational realities such as feed processing, farm labor constraints, and seasonal stress windows continue to shape which solutions scale successfully.As the landscape evolves, policy and trade pressures add a new layer of complexity. Tariff-related cost and supply volatility in 2025 can accelerate changes in sourcing strategies, documentation standards, and the pace of formulation innovation. In response, buyers and suppliers that invest in resilience, traceability, and measurable performance are better positioned to maintain continuity and protect outcomes.
Ultimately, the strongest results come from aligning immunostimulants with integrated health programs rather than viewing them as isolated additives. Organizations that standardize protocols, validate suppliers with real farm data, and tailor strategies by species and region can improve predictability and reduce downside risk. This positions immunostimulants not only as a health tool, but as a strategic lever for operational confidence and market access.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Aquaculture Immunostimulant Market
Companies Mentioned
The key companies profiled in this Aquaculture Immunostimulant market report include:- Alltech Ireland Ltd.
- BIOMIN Holding GmbH
- Cargill, Incorporated
- Huvepharma S.A.
- Kemin Industries, Inc.
- Koninklijke DSM N.V.
- Novozymes A/S
- Novus International, Inc.
- Phibro Animal Health Corporation
- Zoetis Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
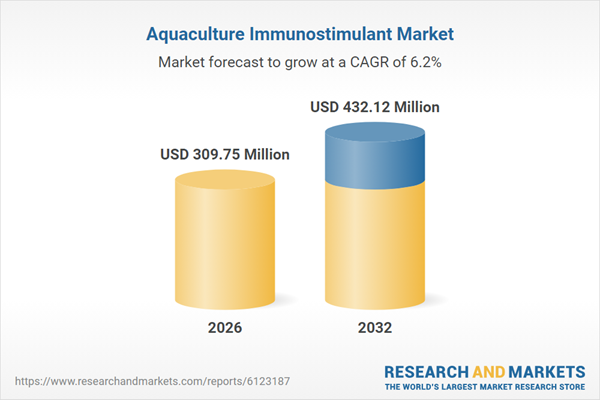
| Estimated Market Value ( USD | $ 309.75 Million |
| Forecasted Market Value ( USD | $ 432.12 Million |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |