Speak directly to the analyst to clarify any post sales queries you may have.
Sterile fill-finish is becoming a strategic control point for quality, speed, and supply resilience as injectable pipelines and scrutiny intensify
Sterile fill-finish sits at the final, most failure-sensitive interface between sterile drug substance and patient-ready dosage form. In an era defined by complex biologics, accelerated development pathways, and heightened scrutiny of aseptic processing, the fill-finish step has become a strategic capability rather than a downstream manufacturing task. Decisions made here directly influence product quality, launch readiness, supply continuity, and ultimately patient safety.The market landscape is being reshaped by a sustained shift in pipelines toward injectables, including biologics, cell and gene therapies, and long-acting formulations. This shift amplifies the need for advanced aseptic techniques, rigorous contamination control strategies, and equipment that supports both small, high-value clinical batches and scalable commercial volumes. At the same time, sponsors and contract manufacturers are expected to meet increasingly demanding expectations for data integrity, process robustness, and real-time release readiness.
Against this backdrop, sterile fill-finish leaders are balancing multiple pressures: the need to de-risk supply chains, secure capacity in the face of demand volatility, and modernize facilities without disrupting ongoing operations. The executive summary that follows frames the most consequential changes in technology, regulation, trade, and partnering models, with the goal of enabling decision-makers to act decisively in a market where delays and deviations can be exceptionally costly.
Technology modernization, barrier-based asepsis, and digital quality systems are redefining fill-finish execution and partner selection criteria
Sterile fill-finish is undergoing a structural modernization driven by both technology and risk management. First, the industry is shifting from legacy cleanroom-heavy models to barrier-centric manufacturing, with isolators and restricted access barrier systems playing a more central role in contamination control. This transformation is not purely compliance-driven; it also supports higher line utilization, more consistent environmental performance, and reduced human intervention, which remains a dominant risk factor in aseptic processing.In parallel, automation and digitalization are changing how operations are executed and governed. Advanced inspection, in-line monitoring, electronic batch records, and equipment connectivity are increasingly used to strengthen deviation detection and accelerate investigations. Importantly, digital maturity is now being evaluated as part of partner qualification, because it affects not only operational efficiency but also audit readiness and the ability to demonstrate control across campaigns, shifts, and sites.
Another transformative shift is the growing prominence of platform-oriented fill-finish services for high-value modalities. Cell and gene therapies, antibody-drug conjugates, and other potent or sensitive products often require dedicated or segregated suites, closed processing, enhanced operator protection, and highly controlled cold-chain handling. This has fueled investment in flexible, smaller-footprint lines optimized for rapid changeovers and low-loss filling, alongside specialized capabilities such as cryogenic handling, single-use flow paths, and advanced container closure integrity approaches.
Finally, partnering models are evolving. Sponsors increasingly pursue dual sourcing, regional redundancy, and earlier engagement with fill-finish providers to align tech transfer timelines with clinical and regulatory milestones. Consequently, providers are differentiating through integrated offerings that connect formulation, analytical services, packaging engineering, and serialization readiness. The competitive edge is shifting toward those who can offer predictable execution across the full lifecycle, from clinical supply through commercial scaling, while maintaining consistent quality systems across networks.
United States tariffs in 2025 may ripple through components, equipment, and sourcing choices, accelerating resilience-focused network redesign
United States tariff actions anticipated in 2025 are poised to influence sterile fill-finish decision-making even when the tariffed items are not the finished medicines themselves. The most immediate impact is expected to be felt through inputs and equipment that underpin aseptic operations, including components, consumables, and specialized machinery that may be sourced globally. When tariffs raise landed costs or introduce administrative friction, procurement teams often respond by requalifying suppliers, increasing safety stocks, or redesigning bill-of-material strategies to preserve continuity.Over time, tariffs can reshape network design by changing the relative attractiveness of regional manufacturing footprints. Sponsors that previously optimized fill-finish on a purely technical or capacity basis may incorporate tariff exposure into total-cost-to-serve models, particularly for products with high component content or complex packaging configurations. This can increase interest in nearshoring certain activities, expanding domestic finishing steps, or using regional hubs that reduce cross-border movement of high-sensitivity materials.
Tariff-driven uncertainty also tends to elevate the value of operational flexibility. Providers that maintain multi-region component qualification, interchangeable container formats, and robust change control processes are better positioned to absorb policy shocks without pausing production. In practice, this can accelerate adoption of standardized components, strengthen demand for suppliers with diversified manufacturing sites, and encourage the use of packaging solutions that can be sourced from multiple approved vendors.
Regulatory and quality considerations remain central, and they interact with trade policy in complex ways. Requalifying a component or changing a supplier can require comparability work, stability data, and regulatory filings depending on the product and market. As a result, tariff pressure may not yield immediate shifts for commercial products but can heavily influence pipeline programs, where development teams can design in flexibility earlier. Overall, the cumulative impact of 2025 tariffs is likely to be less about a single cost increase and more about accelerating strategic moves toward resilient sourcing, simplified qualification pathways, and regionally balanced fill-finish capacity.
Segment dynamics across product types, dosage forms, technologies, containers, and end users reveal where complexity and differentiation concentrate
Segmentation by product type highlights how technical requirements diverge across sterile portfolios. Biologics and advanced therapies tend to heighten sensitivity to shear, temperature excursions, and adsorption, which pushes demand toward low-loss filling, single-use product contact surfaces, and tightly controlled hold times. In contrast, small-molecule injectables often emphasize throughput, container compatibility, and cost-effective batch economics, while still requiring rigorous particulate control and robust sterility assurance.When viewed through the lens of dosage form, liquid fill-finish continues to anchor many commercial operations, but it also exposes sponsors to cold-chain constraints and stability limits that complicate distribution. Lyophilized formats introduce longer cycle times and equipment bottlenecks, yet they can unlock meaningful stability benefits and may reduce shipping risk for temperature-sensitive molecules. Prefilled presentations further shift the decision criteria toward functionality, device integration, and human factors, raising the importance of early collaboration among development, packaging engineering, and manufacturing teams.
Segmentation by process and technology reveals a clear premium on systems that reduce human intervention and strengthen contamination control. Aseptic filling in isolators and barrier systems is increasingly treated as a baseline expectation for many high-risk or high-value products, while terminal sterilization retains relevance where formulation and container systems allow it, due to its inherent sterility assurance advantages. Meanwhile, innovations such as robotic handling, automated weight checks, and enhanced environmental monitoring are becoming differentiators because they influence batch release speed and investigation burden.
Container and closure segmentation underscores how packaging choices cascade into supply chain and quality outcomes. Vials remain a workhorse format, but they require careful management of glass quality, delamination risk where applicable, and container closure integrity. Syringes and cartridges introduce tighter dimensional requirements and more complex assembly considerations, while bags and bottles are critical for certain therapies and diluents. The growing adoption of polymer-based containers and ready-to-use components reflects a push to reduce washing and depyrogenation complexity, though it also increases reliance on supplier quality and incoming inspection rigor.
Finally, segmentation by end user brings a practical view of buying behavior and operational priorities. Innovator pharmaceutical and biotechnology companies increasingly seek partners that can support accelerated timelines, manage tech transfer with disciplined comparability strategies, and sustain quality through scale-up. Contract development and manufacturing organizations prioritize flexible scheduling, multi-client segregation, and platform processes that minimize changeover time. Hospitals, clinics, and other care settings indirectly influence requirements through administration preferences and safety features, which can steer demand toward user-friendly prefilled formats and device-enabled delivery systems.
Regional performance in the Americas, EMEA, and Asia-Pacific reflects differing priorities in compliance rigor, capacity expansion, and supply chain proximity
Regional insights start with the Americas, where a strong base of biologics innovation and an active clinical pipeline continue to drive demand for flexible sterile fill-finish capacity. The region’s priorities increasingly center on quality system maturity, inspection readiness, and capacity assurance for both established injectables and specialized therapies. In addition, supply chain risk management and the desire for geographically closer production are shaping partner selection, particularly for products with cold-chain requirements or high component sensitivity.Across Europe, the Middle East, and Africa, regulatory rigor and cross-border supply considerations shape a diverse operating environment. Western Europe remains a hub for high-compliance aseptic manufacturing, complex packaging, and multi-market release coordination, while certain countries are investing to strengthen local capabilities and reduce dependence on external supply. The region’s mix of mature markets and emerging access needs encourages providers to balance high-end innovation support with cost-effective, reliable operations.
In Asia-Pacific, the landscape is defined by rapid capability expansion, rising quality expectations, and increasing participation in global supply networks. Several markets are scaling advanced fill-finish infrastructure, including barrier systems and modern inspection, to support both domestic demand and export-oriented production. At the same time, sponsors often evaluate regional partners through the lens of governance, data integrity, and consistent performance across sites, especially when products are destined for highly regulated markets.
Taken together, regional segmentation shows that capacity is no longer assessed solely on volume. Instead, sponsors weigh proximity to drug substance manufacturing, resilience against logistics disruption, access to specialized labor, and the ability to navigate local regulatory pathways without introducing avoidable variation. This is driving a more deliberate, portfolio-based approach to regional sourcing that balances speed, risk, and lifecycle flexibility.
Competitive advantage among sterile fill-finish providers increasingly comes from modern aseptic infrastructure, resilient supply chains, and lifecycle-grade execution
Key companies in sterile fill-finish compete on a combination of technical breadth, quality execution, and lifecycle partnership depth. Leaders distinguish themselves by offering multiple filling platforms that span vials, syringes, cartridges, and specialty containers, supported by robust inspection, container closure integrity programs, and packaging engineering expertise. Increasingly, the strongest providers can demonstrate repeatable tech transfer playbooks, disciplined deviation management, and a governance model that scales from clinical to commercial without introducing quality drift.A defining competitive factor is investment in modern aseptic infrastructure. Companies that have expanded isolator-based capacity, implemented high-automation lines, and upgraded utilities and environmental monitoring are better positioned to meet contemporary expectations for contamination control. This advantage becomes particularly visible for high-potency compounds and advanced therapies, where segregation, closed processing, and operator protection requirements narrow the set of qualified partners.
Another differentiator is supply chain orchestration. Providers with strong supplier qualification programs, secure access to ready-to-use components, and validated cold-chain handling can reduce execution risk for sponsors. In addition, firms that operate multi-site networks can offer redundancy strategies, second-line qualifications, and regional options that help mitigate policy shocks, logistics disruptions, or localized capacity constraints.
Finally, customer experience is becoming a measurable dimension of competition. Sponsors value transparency in scheduling, clear deviation narratives, proactive regulatory support, and data systems that allow rapid information exchange. Companies that treat communication cadence, documentation quality, and on-time batch disposition as strategic priorities tend to win repeat business, especially as portfolios become more complex and timelines more compressed.
Leaders can reduce risk and improve speed by managing fill-finish as a portfolio capability, modernizing contamination control, and building sourcing flexibility
Industry leaders can strengthen their sterile fill-finish posture by treating capacity as a strategic asset governed at the portfolio level. Rather than sourcing program by program, organizations should map products by sterility risk, container complexity, temperature sensitivity, and launch criticality, then align each cluster to a differentiated sourcing and technology strategy. This approach supports clearer prioritization of isolator capacity, lyophilization access, and specialized handling for advanced modalities.Operationally, leaders should invest in contamination control as an integrated system that spans facility design, operator practices, environmental monitoring, and maintenance discipline. Upgrading to barrier-based filling where justified, tightening component control, and improving line clearance effectiveness can reduce deviation rates and protect supply continuity. In parallel, expanding digital quality capabilities-such as electronic batch records, automated deviation workflows, and audit-ready data governance-improves both compliance performance and decision speed.
To mitigate tariff and trade uncertainty, organizations should proactively engineer sourcing flexibility. This includes qualifying multiple suppliers for critical components, standardizing container systems across programs when feasible, and negotiating agreements that protect access to constrained materials. For pipeline assets, teams should embed flexibility early by selecting components with broader availability and by planning comparability strategies that reduce friction if substitutions become necessary.
Partnering discipline is equally essential. Leaders should define clear technical and quality gate criteria for provider selection, including demonstrated performance with similar modalities, validated container closure integrity approaches, and proven cold-chain controls. Joint governance models that include shared risk registers, escalation pathways, and regular performance reviews can prevent small issues from becoming launch-threatening events. Where internal investment is considered, decisions should be anchored to long-term portfolio needs, talent availability, and the ability to sustain world-class quality systems over time.
A blended methodology combining expert validation, structured segmentation, and triangulated analysis supports reliable, decision-oriented conclusions
The research methodology integrates qualitative and analytical approaches to build a decision-oriented view of the sterile fill-finish landscape. The work begins with structured secondary research to establish the technical and regulatory context, including aseptic processing expectations, evolving quality practices, packaging trends, and modality-driven requirements. This step also maps the ecosystem of service providers, technology enablers, and component suppliers that influence fill-finish readiness.Primary research is then used to validate assumptions and capture real-world operating conditions. Interviews and structured discussions with stakeholders such as manufacturing leaders, quality professionals, supply chain managers, and packaging specialists help clarify how organizations prioritize risk, what constraints are most common, and which capabilities create meaningful differentiation. These inputs are synthesized to identify consistent themes across modalities and regions, while also highlighting where practices diverge based on regulatory exposure or product mix.
Analytical triangulation is applied to reconcile perspectives and ensure internal consistency across findings. Segmentation frameworks are used to organize insights across product types, dosage forms, process technologies, containers and closures, and end users, enabling comparisons that remain grounded in operational reality. Regional analysis similarly evaluates how compliance expectations, infrastructure maturity, and supply chain considerations shape decision-making.
Throughout the process, quality checks are used to strengthen reliability. Conflicting inputs are assessed through follow-up validation, and conclusions are framed to emphasize practical implications for capacity planning, partner selection, and risk mitigation. The result is a methodology designed to support executive decisions with clear logic, traceable reasoning, and a focus on actionable relevance.
Sterile fill-finish strategy is converging on higher expectations for sterility assurance, partner governance, and resilient supply chains under uncertainty
Sterile fill-finish is moving into a period where operational excellence and strategic foresight carry equal weight. Technology shifts toward barrier systems, automation, and digital quality are raising the baseline for what qualifies as a capable provider, while the growing complexity of injectable pipelines is narrowing tolerance for delays, deviations, and weak governance. At the same time, component availability, cold-chain demands, and policy-driven trade uncertainty are pushing organizations to design for flexibility rather than optimize for a single steady-state scenario.The most successful stakeholders will be those who treat fill-finish as a critical element of product strategy. That means aligning packaging and process choices early, selecting partners based on demonstrated lifecycle performance, and building resilient supply chains that can accommodate substitutions without triggering avoidable regulatory friction. It also means investing in the systems-both technical and organizational-that shorten investigation cycles and strengthen sterility assurance.
Ultimately, the market’s direction is clear: stakeholders are converging on higher standards for contamination control, data integrity, and execution predictability. Organizations that act now to modernize, diversify risk, and govern partnerships tightly will be best positioned to deliver uninterrupted sterile supply to patients while maintaining compliance and operational confidence.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Pharmaceutical Sterile Fill-Finish Market
Companies Mentioned
The key companies profiled in this Pharmaceutical Sterile Fill-Finish market report include:- AbbVie Inc.
- Aenova Group GmbH
- Baxter International Inc.
- Boehringer Ingelheim International GmbH
- Catalent, Inc.
- Delpharm Group
- Fresenius Kabi AG
- Grand River Aseptic Manufacturing, LLC
- Lonza Group AG
- PCI Pharma Services, Inc.
- Pfizer Inc.
- Recipharm AB
- Samsung Biologics Co., Ltd.
- Thermo Fisher Scientific Inc.
- Vetter Pharma-Fertigung GmbH & Co. KG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
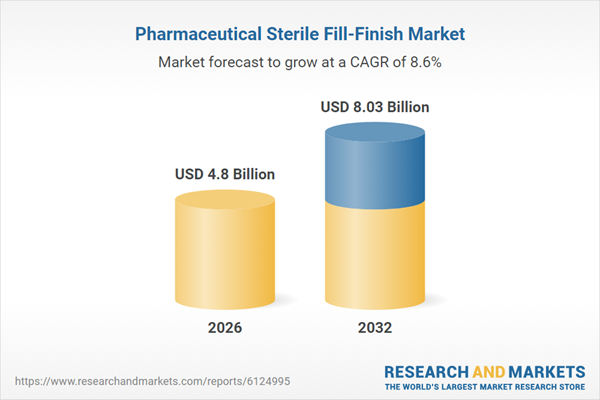
| Estimated Market Value ( USD | $ 4.8 Billion |
| Forecasted Market Value ( USD | $ 8.03 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |