Speak directly to the analyst to clarify any post sales queries you may have.
Adult genetic disease diagnosis is becoming a frontline clinical tool, reshaping care pathways, operational models, and patient expectations across health systems
Adult genetic disease diagnosis has moved from a niche specialty into a core capability for modern healthcare systems. What was once largely confined to rare pediatric conditions now plays a pivotal role in adult-onset disorders, including hereditary cancer syndromes, cardiomyopathies and arrhythmias, familial hypercholesterolemia, neurodegenerative diseases, renal and metabolic disorders, and a widening set of pharmacogenomic use cases. This expansion is driven by converging forces: improved sequencing performance, broader clinician awareness, rising patient demand, and the clinical urgency to identify actionable inherited risk before irreversible disease progression.At the same time, the category is becoming operationally complex. Health systems must balance comprehensive test selection with appropriate utilization, manage counseling capacity and informed consent, and coordinate between primary care, specialists, and laboratory partners. The data layer is equally challenging, as interpretation standards evolve, variant classifications shift over time, and longitudinal reanalysis becomes part of quality care. Consequently, the adult genetic diagnosis landscape increasingly rewards organizations that combine analytical excellence with scalable workflows, robust informatics, and clear clinical pathways.
Against this backdrop, executive decision-makers face a central question: how to expand access to high-value genetic testing while preserving clinical validity, patient trust, and financial sustainability. Understanding where the landscape is shifting, how supply chains and trade policy may influence platform economics, and how segmentation dynamics shape buyer behavior is essential for navigating the next stage of growth.
Transformative shifts are redefining adult genetic diagnosis through broader testing strategies, AI-enabled interpretation, and integrated care delivery models
The landscape is undergoing transformative shifts that go beyond incremental improvements in sequencing. First, test strategy is moving from single-gene or narrow panels toward broader panels and genome-wide approaches when clinical context supports it. This is not simply a technology preference; it reflects the reality that adult phenotypes are often heterogeneous and overlapping. Clinicians increasingly seek higher diagnostic yield when the differential is wide, particularly for cardiogenetics, neurogenetics, and complex multisystem presentations.Second, interpretation is shifting from a laboratory-only function to a distributed, multidisciplinary activity. Molecular tumor boards and cardiovascular genetics case conferences have set a precedent for collaborative review, and similar models are emerging for adult rare disease and neurogenetics. This trend is reinforced by the growing importance of phenotypic data quality, family history structure, and the need to reconcile findings with imaging, pathology, and longitudinal outcomes.
Third, AI-enabled triage and variant interpretation support tools are becoming embedded across the workflow. Rather than replacing expert judgment, these tools are increasingly used to prioritize variants, harmonize evidence across databases, and identify candidates for reclassification monitoring. In parallel, the informatics layer is becoming a strategic asset as organizations aim to integrate genomic results into the electronic health record with computable formats, enabling clinical decision support, cascade testing prompts, and population health outreach.
Fourth, access models are evolving. Remote genetic counseling, hybrid telehealth pathways, and asynchronous education modules are being adopted to address counselor shortages while maintaining informed consent standards. Meanwhile, payer scrutiny remains high, pushing laboratories and health systems to generate stronger clinical utility evidence, reduce unnecessary repeat testing, and document guideline-concordant ordering.
Finally, the market is shifting toward ecosystem partnerships. Instrument vendors, specialty labs, health systems, and digital health firms are forming tighter collaborations around end-to-end solutions that include sample logistics, automation, bioinformatics pipelines, reporting, and clinician education. As these integrated models mature, differentiation is increasingly defined by turnaround time, interpretive clarity, and downstream clinical actionability rather than sequencing alone.
United States tariffs in 2025 introduce cost and supply chain volatility that will shape platform decisions, procurement strategy, and testing economics
United States tariffs taking effect in 2025 add a new layer of strategic pressure to adult genetic diagnostics, particularly where globalized supply chains intersect with regulated clinical operations. Many laboratories and health systems depend on imported components across sequencing instruments, liquid-handling automation, plastics and consumables, optical parts, and certain categories of reagents and chemicals. Even when final assembly occurs domestically, upstream dependencies can expose organizations to cost volatility and lead-time risk.The immediate impact is rarely uniform. Some suppliers may absorb portions of tariff-related increases in the short term to preserve share, while others pass through costs via reagent pricing, service contracts, or updated minimum order quantities. Over time, procurement teams are likely to face more frequent price revisions and shorter quote validity windows. This dynamic complicates budgeting for expanding test menus, scaling capacity, or entering multi-year purchasing agreements, especially for programs that depend on predictable per-sample economics.
Operationally, tariffs can also influence platform choices. Laboratories evaluating new sequencers or automation systems may weigh not only performance and throughput but also the resilience of vendor sourcing, the proportion of domestically produced consumables, and the availability of secondary suppliers. Service and maintenance considerations become more critical if parts logistics are affected by customs delays. Additionally, laboratories may accelerate inventory buffering for key consumables, which can raise working capital requirements and create storage and expiry management challenges.
From a strategic perspective, the tariffs may catalyze domestic manufacturing investments and localized supply chain diversification. However, reshoring is not instantaneous in highly regulated environments, where validation requirements, quality management systems, and lot-to-lot comparability constraints can slow substitution. As a result, the near-term implication for industry leaders is the need for rigorous scenario planning: mapping bill-of-material exposure, identifying single points of failure, negotiating contract protections, and establishing qualification pathways for alternative inputs without compromising assay performance.
Finally, tariff-driven cost pressure can intensify payer negotiations and utilization management. If laboratories attempt to offset higher input costs through pricing adjustments, they may encounter stricter evidence demands. Therefore, aligning procurement strategy with clinical value articulation becomes essential to protect access, maintain margins, and sustain program growth.
Segmentation insights show divergent growth drivers by test type, technology, application, and end-user models as adult genetic services become longitudinal
Key segmentation insights reveal how demand patterns and competitive strategies vary across the adult genetic diagnosis ecosystem. When viewed through the lens of test type, there is continued momentum toward multigene panels for hereditary cancer and cardiovascular indications because they balance breadth with interpretability and manageable reporting complexity. At the same time, exome and genome sequencing are expanding in adult rare disease and undiagnosed conditions, particularly when prior testing has been inconclusive. This shift elevates the importance of phenotype capture, reanalysis policies, and clear communication of secondary findings.Considering technology, next-generation sequencing remains foundational, but differentiation increasingly comes from library preparation efficiency, error correction approaches, and the ability to detect structural variants and repeat expansions where clinically relevant. Complementary methods, including microarrays for specific applications and targeted PCR-based assays for known variants, remain important in streamlined workflows and confirmatory testing. As laboratories broaden menus, they are also prioritizing automation and informatics to reduce hands-on time, minimize batch effects, and improve reproducibility.
From the perspective of application, oncology-related hereditary risk assessment continues to drive structured care pathways, supported by established guidelines and growing clinician familiarity. Cardiovascular genetics is gaining prominence as inherited arrhythmias and cardiomyopathies become more frequently recognized in adult populations, often after sentinel events. Neurology and neurodegeneration represent a complex but expanding arena, where test selection and counseling are nuanced and where clinicians seek clarity on penetrance, variable expressivity, and implications for family members.
Looking at end users, hospitals and integrated delivery networks are investing in in-house capabilities when they have sufficient volume and the need for tight clinical integration. Independent and specialty laboratories remain critical for advanced assays, high-complexity interpretation, and scalable capacity, particularly for health systems that prefer referral models. Academic and research-affiliated centers continue to influence clinical standards through translational programs and novel gene-disease associations, which can later become mainstream clinical offerings.
Finally, segmentation by workflow and service model highlights a growing preference for end-to-end solutions that combine testing with genetic counseling support, prior authorization assistance, and report interpretability tools for clinicians. Organizations that can flex between centralized testing and decentralized ordering, while maintaining consistent quality and patient experience, are positioned to win as adult genetic diagnosis becomes a longitudinal component of care rather than a one-time event.
Regional insights reveal how reimbursement, regulation, infrastructure, and workforce realities shape adult genetic diagnosis adoption across major markets
Regional dynamics in adult genetic disease diagnosis are shaped by healthcare infrastructure, reimbursement norms, workforce availability, and regulatory requirements. In the Americas, adoption is strongly influenced by payer policies, employer-sponsored health dynamics, and the maturity of hereditary cancer programs, with increasing emphasis on integrating results into routine care through electronic records and coordinated specialty referrals. Health systems are also focusing on equity initiatives to address disparities in access and variant interpretation for underrepresented populations.In Europe, the landscape reflects a mix of national health system approaches, with strong emphasis on clinical governance, evidence standards, and data protection. Cross-border variability in reimbursement and testing pathways means suppliers often need country-specific commercialization strategies. At the same time, European programs frequently emphasize standardized guidelines, quality accreditation, and structured pathways for cascade testing, particularly for conditions with clear preventive interventions.
The Middle East & Africa region presents a diverse picture, where centers of excellence are expanding advanced genetic services, often linked to national genomics initiatives and investments in precision medicine. However, access can be uneven due to differences in laboratory infrastructure, specialist availability, and funding mechanisms. As regional capabilities mature, partnerships that include training, technology transfer, and locally relevant variant databases can meaningfully improve diagnostic confidence.
In Asia-Pacific, growth is propelled by expanding healthcare capacity, increasing awareness, and a rising burden of chronic disease where genetic risk stratification has clinical value. Large populations and national-level digital health initiatives create opportunities for scalable programs, yet regulatory and reimbursement variability can shape market entry. Additionally, local manufacturing strengths in some countries can influence platform economics, while regional genomic diversity underscores the need for population-specific reference data.
Across all regions, a unifying theme is the shift from episodic testing toward ongoing genomic care. Regions that can align reimbursement with preventive outcomes, develop counseling capacity through hybrid models, and strengthen data interoperability will be better positioned to translate genetic findings into measurable improvements in adult health.
Company insights highlight competition across labs, platform vendors, and software players as integrated solutions and clinical actionability become decisive
Key company insights emphasize that competitive advantage increasingly depends on end-to-end execution rather than isolated capabilities. Leading diagnostic laboratories are differentiating by expanding clinically curated panels, strengthening variant interpretation frameworks, and offering structured reanalysis programs. Many are also investing in clinician-facing report design that improves actionability, reduces ambiguity, and supports downstream care coordination.Instrument and reagent providers are competing on workflow simplification, throughput flexibility, and reliability under real-world clinical conditions. Beyond headline performance metrics, buyers scrutinize run stability, sample failure rates, contamination controls, and service responsiveness. Vendors that pair hardware with validated assays, robust bioinformatics, and compliance-ready documentation can shorten time-to-implementation for hospital labs and reduce the burden on quality teams.
Digital and software-focused companies are gaining influence through platforms that unify ordering, consent, sample tracking, interpretation support, and results delivery. Interoperability with electronic health records, standards-based data formats, and configurable clinical decision support are increasingly decisive in enterprise purchasing. Companies that can demonstrate secure data handling, auditability, and governance features are better positioned as genomic data becomes a long-lived clinical asset.
Genetic counseling service providers and hybrid care networks represent another important segment, addressing workforce constraints with telehealth delivery, scheduling infrastructure, and educational content that meets patient literacy needs. Their role is expanding from pre-test counseling into post-test navigation, cascade testing coordination, and longitudinal follow-up when variant classifications change.
Across the competitive set, partnership strategy matters. Alliances between labs and health systems, co-development agreements for specialized assays, and collaborations that build population-specific variant resources can accelerate credibility and adoption. Companies that proactively support evidence generation, guideline alignment, and payer engagement are more likely to sustain durable relationships in adult-focused programs where outcomes and utilization are closely monitored.
Actionable recommendations focus on resilient supply chains, evidence-aligned clinical pathways, scalable counseling models, and interoperable genomic data operations
Industry leaders should begin by treating supply chain resilience as a clinical reliability requirement, not merely a procurement concern. This means mapping critical dependencies for instruments, consumables, and key reagents; negotiating contingency clauses in contracts; and qualifying secondary suppliers where validation pathways allow. In parallel, organizations can reduce exposure by standardizing on fewer platforms where appropriate, while maintaining enough redundancy to prevent single points of failure.Next, leaders should prioritize clinical pathway design that aligns test selection with evidence-based indications and clear downstream actions. Embedding ordering guidance into the electronic health record, coupled with decision support that prompts appropriate referrals, can reduce inappropriate utilization and strengthen payer alignment. Where counseling capacity is constrained, hybrid models that combine digital education, triage protocols, and targeted counselor time can preserve informed consent while expanding access.
To improve interpretive quality and efficiency, organizations should invest in a modern interpretation operating model. This includes structured phenotype capture, multidisciplinary case review for complex findings, and defined policies for reanalysis and recontact. Building governance around variant classification-such as audit trails, conflict resolution processes, and periodic review-improves consistency and reduces risk.
Commercial and payer strategies should focus on demonstrating clinical utility in terms decision-makers recognize, including avoided adverse events, optimized therapy selection, and preventive interventions enabled by early detection. Strengthening documentation, prior authorization workflows, and outcomes tracking can reduce friction and denials. Additionally, aligning patient access programs with transparent eligibility criteria helps protect trust while supporting equitable adoption.
Finally, leaders should view data interoperability as a strategic lever. Ensuring results are captured in computable formats, enabling automated prompts for cascade testing, and supporting longitudinal monitoring can transform genetic diagnosis from a static report into an ongoing care capability. This transition positions organizations to deliver measurable value as precision medicine becomes operationalized across adult specialties.
Research methodology blends expert interviews with rigorous secondary review and triangulation to reflect real-world clinical, technical, and policy conditions
The research methodology for this executive summary is grounded in a structured approach to understanding adult genetic disease diagnosis across clinical workflows, technology choices, buyer behavior, and policy influences. The process begins with defining the scope of adult-focused genetic applications and establishing consistent terminology for test modalities, interpretation practices, and service models to ensure comparability across stakeholders.Primary research typically incorporates interviews and structured discussions with a cross-section of knowledgeable participants, such as laboratory directors, clinical geneticists, genetic counselors, pathologists, cardiologists, neurologists, oncology program leads, procurement and supply chain managers, and executives responsible for diagnostics strategy. These conversations are used to validate real-world pain points, adoption barriers, and decision criteria, with careful attention to differences between hospital-based programs, reference laboratories, and hybrid delivery networks.
Secondary research generally reviews publicly available materials including regulatory guidance, professional society recommendations, payer coverage policies, company product documentation, peer-reviewed clinical literature, and public disclosures related to manufacturing and supply chain updates. This step helps establish the current state of technology, quality standards, and compliance expectations, while identifying emerging themes such as AI-assisted interpretation, data governance, and remote counseling models.
Triangulation is applied by cross-validating themes across multiple inputs, reconciling discrepancies through follow-up checks, and prioritizing findings that are consistent across independent viewpoints. Qualitative synthesis is then used to organize insights into strategic narratives that link technology and workflow choices to clinical value, operational feasibility, and policy constraints. Throughout the process, the emphasis remains on actionable understanding rather than speculative claims, ensuring the final analysis supports practical decision-making.
Conclusion underscores that scalable integration, interpretive governance, and operational resilience will define success in adult genetic disease diagnosis
Adult genetic disease diagnosis is entering a phase where maturity is defined by execution, integration, and trust. The core technologies are advancing, but the more decisive differentiators now lie in how effectively organizations embed genetic insights into clinical pathways, maintain interpretive rigor at scale, and deliver an experience that supports patients and clinicians through complex decisions.As the landscape shifts toward broader testing, AI-assisted workflows, and integrated service models, leaders must balance innovation with governance. The ability to manage reanalysis, ensure data security, and maintain consistent variant interpretation will increasingly influence credibility and clinical adoption. Meanwhile, workforce constraints and payer scrutiny reinforce the need for efficient care models that still meet high standards for consent, counseling, and documentation.
Looking ahead, external pressures such as tariff-driven supply chain variability further elevate the importance of resilient operations and strategic procurement. Organizations that proactively align platform strategy, clinical value articulation, and interoperable data infrastructure will be better positioned to expand access responsibly. Ultimately, the category’s direction is clear: genetic diagnosis in adults is becoming longitudinal, system-wide, and outcome-oriented, demanding leadership that can coordinate across technology, clinical practice, and policy realities.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Adults Genetic Disease Diagnosis Market
Companies Mentioned
The key companies profiled in this Adults Genetic Disease Diagnosis market report include:- Abbott Laboratories
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Centogene N.V.
- F. Hoffmann-La Roche Ltd.
- Illumina, Inc.
- Invitae Corporation
- Myriad Genetics, Inc.
- Natera, Inc.
- QIAGEN N.V.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 187 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
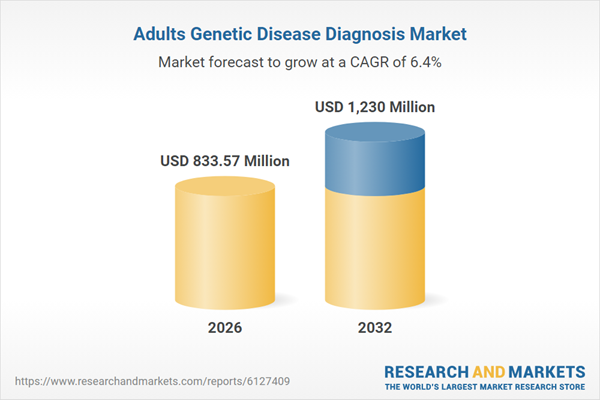
| Estimated Market Value ( USD | $ 833.57 Million |
| Forecasted Market Value ( USD | $ 1230 Million |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |