Speak directly to the analyst to clarify any post sales queries you may have.
The Mirabegron Drugs Market offers significant commercial opportunities amid a shifting regulatory environment, competitive expansion, and technological integration in overactive bladder management. This report delivers actionable intelligence for senior decision-makers evaluating investment, product development, and partnership options in this dynamic field.
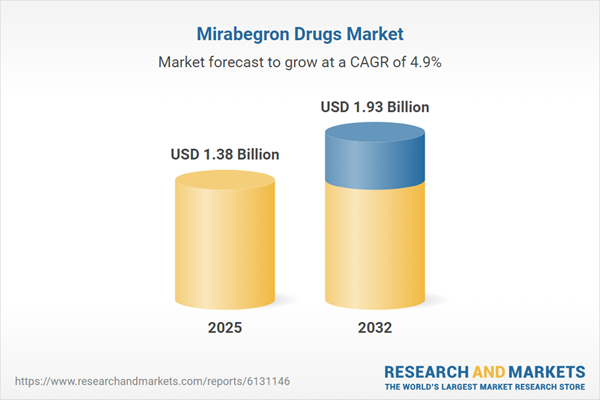
Market Snapshot: Mirabegron Drugs Market Growth and Outlook
The Mirabegron Drugs Market grew from USD 1.31 billion in 2024 to USD 1.38 billion in 2025. It is expected to continue growing at a CAGR of 4.91%, reaching USD 1.93 billion by 2032. This progression is propelled by Mirabegron's differentiated therapeutic profile in overactive bladder management, mounting competition from generics, evolving regulatory policies, and the rapid uptake of digital health solutions. Market expansion is also driven by changing patient demographics and ongoing shifts in supply chain resilience and production strategies.
Scope & Segmentation
This comprehensive research covers the full spectrum of market dynamics influencing Mirabegron's adoption, differentiation, and access. The analysis includes:
- Indication: Idiopathic and neurogenic overactive bladder
- Therapy Type: Combination therapies (with alpha-blocker, with antimuscarinic), Monotherapy
- Formulation: Extended-release tablets, granules for oral suspension
- Dosage Strength: 25 mg and 50 mg tablets
- Product Type: Branded and generic formulations
- Age Group: Adult, geriatric, and pediatric populations
- Packaging: Oral suspension, tablets (blister pack, bottle)
- Distribution Channel: Hospital pharmacies (government and private), online pharmacy, retail pharmacy (chain, independent)
- End User: Clinics, home care, and hospitals
- Geographic Coverage: Americas (US, Canada, Latin America), Europe, Middle East, Africa, and Asia-Pacific (including major and emerging markets such as China, India, and Japan)
- Company Coverage: Key stakeholders include Astellas Pharma Inc., Lupin Limited, Sandoz AG, Sun Pharmaceutical Industries Ltd., Zydus Lifesciences, and others active in development, manufacturing, and supply chain robustness for Mirabegron.
Key Takeaways: Strategic Insights for Senior Decision-Makers
- Mirabegron's innovative mechanism and improved tolerability are leading to growing adoption and greater integration into patient-centered care protocols.
- Generic market entry and patent expirations are intensifying competition, compelling manufacturers to leverage life-cycle management and clinical differentiation strategies.
- The advancement of digital health technologies, including real-time adherence monitoring and outcome tracking, is reshaping engagement models and augmenting value propositions for providers.
- Increasing regulatory focus on value-based contracting and evidence generation is placing pressure on all players to deliver robust real-world outcomes and cost-effectiveness.
- Distinct market dynamics in dosage forms, strength, and distribution channels support segmentation-based resource allocation and market penetration approaches.
- Demand variances across regions highlight the necessity of localized access, partnership models, and customized commercialization strategies.
Tariff Impact on the Mirabegron Value Chain
Recent United States tariffs introduced in 2025 have impacted sourcing, manufacturing, and pricing strategies in the Mirabegron value chain. Stakeholders are building resilience through expanded supplier networks, optimized inventory practices, and consideration of nearshoring or regional production facilities. These cost and sourcing shifts are prompting ongoing assessments to minimize risk and support uninterrupted patient access.
Methodology & Data Sources
This report is based on a layered methodology, incorporating interviews with industry leaders, healthcare authorities, and supply chain experts. Desk research includes the latest regulatory filings, peer-reviewed publications, and proprietary market databases. Advanced analytical frameworks such as SWOT analysis and multi-criteria segmentation clarify competitive and clinical developments.
Why This Report Matters
- Provides decision-makers with a granular view of the Mirabegron market’s structure, current challenges, and future growth corridors.
- Enables effective portfolio and investment decisions by mapping competitive dynamics, clinical innovation, and regional access challenges.
- Equips executive teams with robust evidence and scenario planning tools to drive strategic action and mitigate emerging risks.
Conclusion
This research delivers proven insights to guide senior executives in navigating the evolving Mirabegron landscape. Stakeholders who act on this intelligence can optimize strategies, strengthen market presence, and respond effectively to regulatory and competitive change.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Mirabegron Drugs market report include:- Astellas Pharma Inc.
- Lupin Limited
- Zydus Lifesciences Limited
- Sandoz AG
- Sun Pharmaceutical Industries Ltd.
- Cipla Ltd.
- Glenmark Pharmaceuticals Limited
- Alembic Pharmaceuticals Ltd
- Ipca Laboratories Ltd
- Intas Pharmaceuticals Ltd
- MSN Laboratories Private Limited
- Alkem Laboratories Ltd.
- Ajanta Pharma Limited
- Dr. Reddy's Laboratories Limited
- Minakem SAS
- STERIS HEALTHCARE PVT LTD
- Strides Pharma Science Limited
- Eskayef Pharmaceuticals Limited
- Neuland Laboratories Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.38 Billion |
| Forecasted Market Value ( USD | $ 1.93 Billion |
| Compound Annual Growth Rate | 4.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |