Key Market Trends and Insights
- Stress incontinence held the largest share of around 30.03% during the historical period.
- Anticholinergics dominate the market by drug type.
- The female segment leads the market by gender, reflecting higher prevalence and treatment rates.
- Hospital pharmacies represent the leading distribution channel in the market.
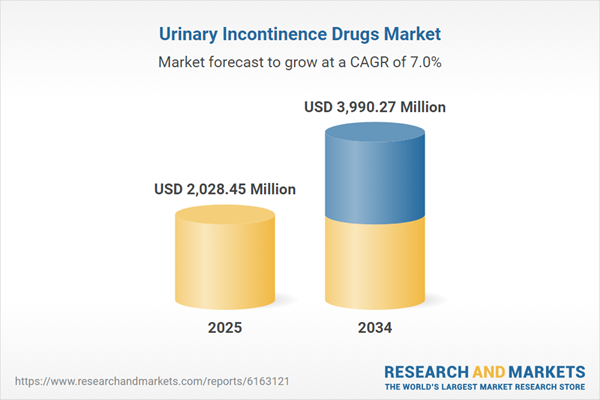
Market Size and Forecast
- Market Size (2024) : USD 2.02 billion
- Projected Market Size (2034) : USD 3.99 billion
- CAGR (2025-2034) : 7.00%
- Largest Market in 2024 : United States
Urinary Incontinence Drugs Market Overview
Urinary incontinence drugs are pharmaceutical treatments designed to manage involuntary urine leakage by improving bladder control and reducing urinary urgency. These medications include anticholinergics, beta-3 adrenergic agonists, and hormone therapies, commonly used for stress, urge, and mixed incontinence. The market is poised to grow at a CAGR of 7.00% during the forecast period of 2025-2034, driven by the rising geriatric population, increasing prevalence of chronic conditions like diabetes and obesity, and growing awareness about urinary health. Advancements in drug formulations and expanding access to healthcare further support market expansion.Urinary Incontinence Drugs Market Growth Drivers
Generic Drug Penetration to Strengthen Market Growth
The rising demand for affordable and effective therapies continues to drive growth in the urinary incontinence drugs market across the eight major regions. For instance, in June 2019, Lupin received U.S. FDA approval for its Mirabegron Extended-Release Tablets, 25 mg and 50 mg, indicated for overactive bladder (OAB) with symptoms such as urge urinary incontinence and urgency. Following this, in April 2024, Lupin launched Mirabegron Extended-Release Tablets, 25 mg in the United States, after final FDA approval, as a generic version of Astellas Pharma's Myrbetriq®. This strategic development strengthens treatment access and reinforces the growing role of generics in global market expansion.Urinary Incontinence Drugs Market Trends
The market is witnessing several trends including expanding indications for beta-3 agonists and rapidly aging population, among others.Expanding Indications for Beta-3 Agonists to Elevate the Market Value
The market is increasingly driven by the expansion of approved indications for beta-3 adrenergic receptor agonists, targeting broader patient populations. In May 2024, Sumitomo Pharma America advanced this trend by submitting a supplemental New Drug Application (sNDA) for vibegron (GEMTESA®) to the U.S. FDA, aiming to treat overactive bladder symptoms such as urge urinary incontinence, urgency, and urinary frequency, in men receiving pharmacological therapy for benign prostatic hyperplasia. Supported by successful Phase 3 results, this strategic development highlights a shift toward more tailored and combination-targeted therapies, reinforcing the upward trajectory of market growth.Rapidly Aging Global Population Expected to Boost Urinary Incontinence Drugs Market Demand
The growing prevalence of an aging population is emerging as a key factor in the expansion of the urinary incontinence drugs market. This trend reflects the increasing need for effective bladder control therapies as older individuals face higher risks of incontinence. In October 2023, the World Health Organization (WHO) highlighted tha t the population aged 60 years and older will double by 2050, reaching 2.1 billion, with the number of individuals aged 80 years and above projected to triple to 426 million. Furthermore, the Population Reference Bureau reported that the number of Americans aged 65 and older will rise from 58 million in 2022 to 82 million by 2050, increasing their share of the population from 17% to 23%. These developments underscore the expanding patient base and are expected to significantly propel the urinary incontinence drugs market forward.Urinary Incontinence Drugs Market Share
Stress Incontinence to Lead the Market Segmentation Based on Incontinence Type
The market, by incontinence type, is categorized into urge incontinence, overflow incontinence, stress incontinence, mixed incontinence, and others. Among these, the stress incontinence segment is projected to lead the market in the coming years. This trend is primarily driven by the high prevalence of stress urinary incontinence (SUI), particularly among aging women, and the growing demand for safer, non-surgical treatment alternatives. In July 2024, a clinical trial published in The Journal of Urology® introduced TAS-303, an investigational drug that demonstrated significant efficacy and safety in treating women with SUI. Unlike other drugs, which are associated with gastrointestinal and neurological side effects, TAS-303 is a highly selective noradrenaline reuptake inhibitor that avoids these adverse reactions. The study showed a 58% reduction in SUI episode frequency in the TAS-303 group, with benefits observed as early as four weeks into treatment. Such promising developments are expected to reinforce the dominance of the stress incontinence segment, supported by an aging global population and the increasing preference for pharmacological therapies over surgical procedures.Urinary Incontinence Drugs Market Analysis by Region
The report covers key regions including the United States, United Kingdom, Germany, France, Italy, Spain, Japan, and India, with market expansion driven by an aging population and evolving regulatory frameworks. The United States is expected to lead the market due to its high disease prevalence and supportive regulatory environment. According to Stephen W. Leslie et al. (2024), approximately 13 million individuals in the U.S. are affected by urinary incontinence, with prevalence rates exceeding 75% among long-term care residents. Regulatory advancements also support market growth, for example, in December 2024, the U.S. FDA approved GEMTESA® (vibegron) by Sumitomo Pharma America, the first β3 agonist approved for men with overactive bladder symptoms u ndergoing treatment for benign prostatic hyperplasia. These developments underscore the region's momentum in addressing unmet needs and advancing therapeutic options.Leading Players in the Urinary Incontinence Drugs Market
The key features of the market report comprise clinical trials analysis, patent analysis, grant analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Pfizer, Inc.
Pfizer Inc., a leading global biopharmaceutical company, actively contributes to the market through its product TOVIAZ® (fesoterodine fumarate). Approved for the treatment of overactive bladder in adults and neurogenic detrusor overactivity in pediatric patients, TOVIAZ® is available as extended-release tablets in 4 mg and 8 mg strengths. With ongoing updates and rigorous medical review, Pfizer strengthens its presence across major regions by addressing both adult and pediatric incontinence treatment needs.AbbVie
Astellas Pharma, a global leader in urology, has maintained a strong presence in the market across key regions through its well-established treatments for overactive bladder (OAB) and lower urinary tract symptoms (LUTS) linked to benign prostatic hyperplasia (BPH). With a focus on improving quality of life, Astellas continues to expand its OAB portfolio and awareness initiatives, including annual campaigns and digital tools like OAB.ie, reinforcing its commitment to underserved patient needs.Merck KGaA
Merck is actively involved in the urinary incontinence drugs market. MSD offers a broad portfolio addressing various types of incontinence, such as oxybutynin, solifenacin, tolterodine, and mirabegron, which target detrusor overactivity. These drugs support the company's commitment to advancing treatment for overactive bladder and related conditions through evidence-based therapies and global medical outreach.Kissei Pharmaceutical
Kissei Pharmaceutical Co., Ltd., launched Beova® Tablets 50mg in Japan for the treatment of overactive bladder (OAB), a key segment in the urinary incontinence drugs market. Approved in 2018, Beova®, containing vibegron, is a once-daily β3-adrenergic receptor agonist that effectively reduces urinary urgency, frequency, and urge urinary incontinence. This strategic entry strengthens company's urology portfolios and contributes to addressing the rising global burden of OAB, particularly in aging populations across major regions.Other key players in the market are Astellas Pharma, Sanofi S.A., Kyorin Holding, Urovant Sciences, Teva Pharmaceutical Industries Ltd., Eli Lilly and Company, and Ferring Pharmaceuticals.
Urinary Incontinence Drugs Market Segmentation
Urinary Incontinence Drugs Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Incontinence Type
- Urge Incontinence
- Overflow Incontinence
- Stress Incontinence
- Mixed Incontinence
- Others
Market Breakup by Drug Type
- Anticholinergic
- Beta-3 Adrenoceptor Agonists
- Antidepressants
- Alpha Blockers
- Estrogen
- Desmopressin
- Others
Market Breakup by Gender
- Female
- Male
Market Breakup by Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online pharmacies
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Key Questions Answered in the Urinary Incontinence Drugs Market
- What was the urinary incontinence drugs market value in 2024?
- What is the urinary incontinence drugs market forecast outlook for 2025-2034?
- What major factors aid the demand for the urinary incontinence drugs market?
- How has the market performed so far, and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- What are the major urinary incontinence drugs market trends?
- Which incontinence is projected to lead the market segment?
- Which drug type is expected to dominate the market segment?
- Which gender is anticipated to drive the market segment?
- Which distribution channel is likely to dominate the market segment?
- Who are the key players in the urinary incontinence drugs market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer, Inc.
- Astellas Pharma
- AbbVie
- Merck KGaA.
- Sanofi S.A.
- Kissei Pharmaceutical
- Kyorin Holding
- Urovant Sciences
- Teva Pharmaceutical Industries Ltd.
- Eli Lilly and Comp any
- Ferring Pharmaceuticals
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 2028.45 Million |
| Forecasted Market Value ( USD | $ 3990.27 Million |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |