In addition, the rising number of samples being tested has led to the adoption of more advanced ATP assay systems that support faster and more efficient processing. Improvements in preparing, measuring, and managing samples have helped laboratories work more effectively and reduce delays. These changes benefit areas such as drug testing and contamination detection, where time and accuracy matter. In December 2024, Hygiena acquired Nexcor Food Safety Technologies Inc., strengthening its range of ATP and environmental monitoring products and supporting the growing demand for quick, reliable testing solutions.
Moreover, the U.S. ATP Assays market is witnessing a notable shift toward integrated and automated detection systems, particularly in pharmaceutical manufacturing and food quality control. These platforms are designed to streamline contamination monitoring and regulatory compliance by combining rapid testing capabilities with digital record-keeping and cloud-based analytics. The growing need for standardized, traceable, and high-throughput workflows drives adoption across various sectors. This shift aligns with broader trends emphasizing real-time data, operational efficiency, and adherence to evolving regulatory requirements, reinforcing the continued expansion of the U.S. ATP Assays market.
Furthermore, growing awareness of biosafety and quality assurance standards across healthcare, food production, and environmental sectors supports sustained investment in ATP assay technologies. Laboratories and production facilities are placing increased emphasis on minimizing contamination risks and meeting regulatory guidelines set by agencies such as the FDA and USDA. This has led to greater integration of ATP assays within quality control frameworks, particularly within environmental monitoring and routine screening workflows. With organizations prioritizing compliance and efficiency, the U.S. ATP Assays market is expected to benefit from stable, cross-sector demand for fast, accurate, and scalable testing solutions.
U.S. ATP Assays Market Report Segmentation
This report forecasts revenue growth and provides an analysis on the latest trends in each of the sub-segments from 2021 to 2033. For the purpose of this report, the analyst has segmented the U.S. ATP assays market on the test type,application, and end use:Type Outlook (Revenue, USD Million, 2021 - 2033)
- Luminometric ATP Assays
- Enzymatic ATP Assays
- Bioluminescence Resonance Energy Transfer (BRET) ATP Assays
- Cell-based ATP Assays
- Others
Application Outlook (Revenue, USD Million, 2021- 2033)
- Drug Discovery and Development
- Clinical Diagnostics
- Environmental Testing
- Food Safety and Quality Testing
- Others
End Use Outlook (Revenue, USD Million, 2021 - 2033)
- Pharmaceutical and Biotechnology Companies
- Academic and Research Institutes
- Hospital and Diagnostics Laboratories
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific
- Promega Corporation
- PerkinElmer Inc.
- Becton, Dickinson and Company (BD)
- Lonza Group Ltd.
- Danaher Corporation
- Abcam plc
- Quest Diagnostics Incorporated
- Biomerieux SA
- 3M Company
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | July 2025 |
| Forecast Period | 2024 - 2033 |
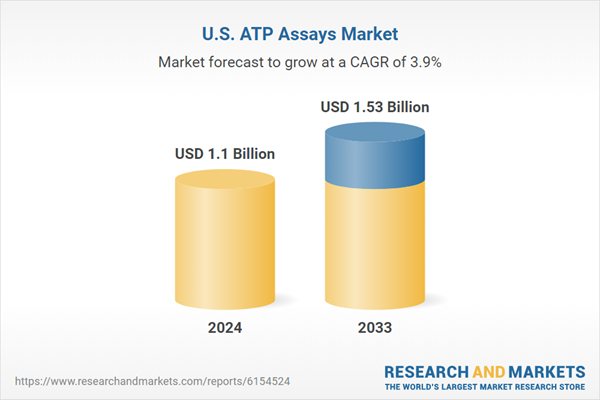
| Estimated Market Value ( USD | $ 1.1 Billion |
| Forecasted Market Value ( USD | $ 1.53 Billion |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |