Speak directly to the analyst to clarify any post sales queries you may have.
A concise and authoritative introduction explaining the technical advantages, laboratory compatibility, and operational rationale driving adoption of protein-free blocking buffers
Protein-free blocking buffers have emerged as a pivotal reagent class in modern immunoassays and molecular biology workflows, offering a reduced risk of cross-reactivity and enhanced reproducibility for sensitive detection techniques. These buffers replace protein-based blockers with synthetic or polymeric components designed to occupy nonspecific binding sites on assay surfaces, thereby minimizing background noise and preserving analyte signal integrity. As laboratories increasingly prioritize assay sensitivity, lot-to-lot consistency, and compatibility with diverse detection chemistries, protein-free formulations have garnered attention across both research and diagnostic settings.Moreover, their adoption is driven by compatibility advantages in multiplexed assay formats and in workflows where endogenous proteins in samples might interfere with detection. Transitioning to protein-free blockers can streamline reagent inventories and simplify validation processes, particularly when the buffers are available in both concentrated and ready-to-use formats that cater to different throughput and automation requirements. Consequently, stakeholders from academic laboratories to clinical diagnostic facilities are appraising protein-free blockers not merely as an alternative chemistry but as a strategic reagent that can reduce assay variability and support robust method transfer. In this context, understanding the technical characteristics, application fit, and procurement considerations of these buffers is essential for procurement managers, research leaders, and product developers who aim to optimize assay performance without introducing confounding protein matrices.
How technological innovation, procurement evolution, and regulatory expectations are driving a strategic transformation in blocking reagent development and supplier practices
The landscape for blocking reagents is undergoing transformative shifts driven by technological advances, evolving assay complexity, and heightened quality expectations across translational research and clinical diagnostics. As detection platforms move toward higher sensitivity and multiplex capacity, end users are demanding blocker chemistries that introduce minimal background, exhibit strong lot-to-lot stability, and are compatible with fluorescence, chemiluminescence, and chromogenic readouts. Consequently, product innovation has focused on refined polymeric formulations and synthetic additives that perform consistently across a wider range of surfaces and membranes.In parallel, changes in procurement and distribution channels are reshaping how laboratories access reagents. Increasing procurement centralization in large academic medical centers and the growth of specialized distributors influence lead times and inventory strategies. At the same time, digital procurement platforms and e-commerce channels have improved accessibility for smaller laboratories, accelerating trial adoption of newer reagent classes. Regulatory scrutiny around diagnostic reagents and a growing emphasis on traceability and quality documentation have prompted suppliers to provide more comprehensive technical dossiers, validated protocols, and reproducibility data. Together, these shifts are fostering an environment where scientific performance, supply reliability, and documentation transparency are equally valued, prompting manufacturers and suppliers to adapt commercial and technical strategies accordingly.
Assessment of how recent tariff adjustments and trade policy dynamics have reshaped reagent sourcing strategies, supply resilience, and procurement risk management for laboratories
Recent tariff actions and trade policy changes have introduced new layers of complexity into global reagent supply chains, affecting raw material sourcing, import logistics, and pricing dynamics. Tariffs can increase landed costs for specialized polymers, surfactants, and proprietary additives that are often produced in concentrated supply geographies, thereby prompting manufacturers to reassess sourcing strategies and supplier diversification. In turn, some producers may shift component sourcing to regional suppliers to mitigate tariff exposure, which can improve lead-time resilience but may require additional qualification and validation of incoming materials.Additionally, changes in tariffs have placed renewed emphasis on inventory planning and contractual terms with distributors. To manage exposure, manufacturers and distributors may opt for hedging strategies, long-term supply agreements, or the reconfiguration of manufacturing footprints to regions with more favorable trade arrangements. These adjustments often cascade downstream, influencing procurement cycles for research institutions and diagnostic laboratories that depend on consistent reagent availability. Importantly, tariff-driven cost pressures can accelerate interest in alternative formulations or in-house buffer preparation where feasible, though such substitutions must be balanced against the technical risk of introducing variability. Therefore, stakeholders should integrate trade-policy monitoring into their sourcing risk assessments and validation plans to preserve assay continuity and maintain high quality standards.
Insightful segmentation analysis that links product formats, assay applications, end-user profiles, and distribution channels to practical reagent selection and procurement decisions
A granular understanding of product and application segmentation is essential for aligning reagent selection with laboratory objectives. From a formulation perspective, the market differentiates between concentrated blocking buffers and ready-to-use blocking buffers, where concentrated formats allow flexible dilution and inventory management for higher-volume workflows while ready-to-use formats prioritize convenience and reduced preparation variability for point-of-care or low-throughput laboratories. When considering application fit, performance demands vary across ELISA, immunohistochemistry, and western blotting; within ELISA, subtypes such as competitive ELISA, indirect ELISA, and sandwich ELISA impose distinct requirements for blocker compatibility with capture and detection chemistries, whereas immunohistochemistry workflows diverge between chromogenic detection and fluorescence detection techniques that have differing sensitivities to background fluorescence and autofluorescence suppression. In western blotting, membrane selection-whether nitrocellulose membrane or PVDF membrane-also informs blocker choice, as membrane porosity and binding characteristics influence nonspecific interactions and signal-to-noise outcomes.End-user segmentation similarly shapes product positioning and support requirements. Academic and research institutes, encompassing both government laboratories and universities, prioritize reproducibility, protocol transparency, and cost-effective options suitable for method development, while biotechnology and pharmaceutical companies focus on scalable, GMP-aligned supplies and robust lot documentation for downstream validation. Diagnostic laboratories and hospitals emphasize regulatory compliance, batch traceability, and vendor support for method transfer under clinical workflows. Distribution channels impact accessibility and procurement experience: offline channels provide hands-on distributor relationships and consolidated procurement for large institutional buyers, whereas online channels offer rapid access, standardized listings, and convenience for smaller labs or ad hoc purchases. Consequently, suppliers and buyers should match format, application compatibility, and distribution pathways to operational priorities to ensure fit-for-purpose reagent deployment.
Regional dynamics and supply chain considerations that reveal how procurement, regulation, and manufacturing footprints shape reagent accessibility and adoption across global markets
Regional dynamics influence supply chains, regulatory requirements, and adoption patterns for protein-free blocking buffers in distinct ways across major global markets. In the Americas, robust academic and industrial research ecosystems drive demand for versatile blocker chemistries and emphasize rapid availability and technical support, with procurement often coordinated through centralized purchasing teams or specialized distributors. This region’s diverse user base also fosters early adoption of novel formulations that demonstrate clear performance benefits in high-throughput or clinical-adjacent applications.Conversely, Europe, the Middle East & Africa present a mosaic of regulatory environments and procurement practices where demand is shaped by stringent diagnostic regulations in certain jurisdictions and by resource considerations in others. As a result, suppliers operating in this region often prioritize comprehensive documentation, localized regulatory support, and distribution partnerships that can manage cross-border logistics. Meanwhile, in the Asia-Pacific region, manufacturing capacity and reagent production footprint are significant factors; localized production can reduce lead times and support competitive pricing, while rapidly expanding research and diagnostic infrastructure in several markets increases demand for both standardized and specialized blocking solutions. Taken together, these regional patterns underscore the importance of tailoring supply, regulatory engagement, and technical services to local laboratory requirements and logistics realities.
Analysis of competitive forces and supplier capabilities that determine market positioning, technical credibility, and distribution effectiveness for reagent manufacturers
Competitive dynamics in the reagent sector are increasingly influenced by product differentiation, technical support capabilities, and supply chain transparency. Leading suppliers compete on the basis of formulation performance, the breadth of validated protocols for different assay types, and the availability of technical application notes that facilitate method transfer across laboratories. In addition, the capacity to provide robust lot-to-lot documentation, stability data, and compatibility matrices with common detection chemistries strengthens vendor credibility among regulated diagnostic users.Strategic partnerships and distribution agreements play a pivotal role in expanding geographic reach and ensuring dependable supply. Companies that maintain regional inventory hubs and offer responsive field application support are often favored by institutional purchasers that require rapid troubleshooting and validation assistance. Moreover, the emergence of contract manufacturing and private-label arrangements allows smaller brands to scale distribution while leveraging established production capabilities. Ultimately, vendors that align product innovation with transparent quality systems, localized logistics, and proactive technical engagement are better positioned to meet the heterogeneous needs of research, industrial, and clinical customers.
Actionable strategic priorities for manufacturers and suppliers to strengthen product validation, supply resilience, digital procurement, and customer support across laboratory segments
Industry leaders should adopt a multi-faceted strategy that balances product excellence, supply resilience, and customer-centric support to capitalize on growing demand for reliable blocking solutions. First, prioritize the development of validated application notes and cross-platform compatibility data that address specific assay subtypes such as competitive, indirect, and sandwich ELISAs, chromogenic and fluorescence immunohistochemistry workflows, and membrane-specific western blot protocols. This technical documentation reduces buyer friction during validation and accelerates method adoption. Second, diversify raw material sourcing and consider regional manufacturing or inventory hubs to mitigate exposure to trade policy fluctuations and to improve lead-time responsiveness for institutional buyers.Third, invest in digital engagement tools and e-commerce capabilities that simplify procurement for smaller laboratories while preserving contract and bulk-order pathways for large institutional purchasers. Fourth, strengthen post-sale technical support and offer modular training or application troubleshooting services to reduce adoption risk and build long-term customer relationships. Finally, align quality systems and documentation practices with clinical and regulatory expectations to serve diagnostic laboratories and hospitals that require traceability and validated supply chains. By executing on these priorities, manufacturers and distributors can enhance product adoption, reduce procurement friction, and improve resilience against supply chain disruptions.
Transparent research methodology describing the qualitative and document-based approach used to validate technical performance, distribution practices, and end-user requirements
A rigorous research methodology underpins the insights presented, combining primary qualitative interviews with end users and technical experts, structured analysis of supplier product literature, and cross-validation through laboratory protocol reviews. Primary engagement with laboratory directors, procurement leads, and application scientists provided firsthand perspectives on assay requirements, validation challenges, and procurement preferences. These qualitative inputs were supplemented by systematic reviews of technical datasheets, compatibility matrices, and peer-reviewed protocols to assess performance claims and identify common validation needs across ELISA, immunohistochemistry, and western blotting workflows.In addition, supplier distribution strategies and logistical practices were evaluated through a review of publicly available distribution channel information and trade publications to understand how offline and online channels affect access and delivery timelines. Throughout the process, findings were corroborated across multiple independent sources to ensure consistency and robustness. The methodological approach emphasizes transparency, reproducibility, and the contextualization of technical and commercial factors to support pragmatic decision-making for procurement, product development, and laboratory operations.
Concluding synthesis that integrates scientific, procurement, and supply chain considerations to guide practical adoption and long-term reliability of blocking reagent choices
As assay sensitivity and multiplex demands continue to grow, protein-free blocking buffers represent a practical reagent class that can address background reduction and reproducibility challenges across diverse laboratory environments. The convergence of technical performance needs, evolving procurement channels, and supply chain considerations requires stakeholders to weigh formulation fit, application compatibility, and distribution responsiveness when selecting blockers. By focusing on validated application guidance, robust documentation, and supply resilience, organizations can reduce assay variability and streamline method transfers from research into diagnostic or production settings.Looking ahead, continued collaboration between reagent developers and end users will be essential to refine formulations that meet the nuanced demands of modern detection chemistries and membrane substrates. Moreover, proactive supply chain planning and targeted technical support will enable laboratories to adopt new chemistries with confidence, ensuring that assay performance improvements translate into meaningful operational gains. In sum, a strategic combination of scientific rigor and pragmatic procurement planning will best position laboratories and suppliers to achieve reproducible, high-quality results.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Type
- Concentrated Blocking Buffers
- Ready-to-Use Blocking Buffers
- Application
- ELISA
- Competitive ELISA
- Indirect ELISA
- Sandwich ELISA
- Immunohistochemistry
- Chromogenic Detection
- Fluorescence Detection
- Western Blotting
- Nitrocellulose Membrane
- PVDF Membrane
- ELISA
- End User
- Academic and Research Institutes
- Government Laboratories
- Universities
- Biotechnology & Pharmaceutical Companies
- Diagnostic Laboratories
- Hospitals
- Academic and Research Institutes
- Distribution Channel
- Offline
- Online
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- Thermo Fisher Scientific Inc.
- Merck KGaA
- Danaher Corporation
- Bio-Rad Laboratories, Inc.
- PerkinElmer, Inc.
- Abcam plc
- Becton, Dickinson and Company
- Agilent Technologies, Inc.
- QIAGEN N.V.
- Takara Bio Inc.
- Advansta Inc.
- Avantor, Inc.
- Azure Biosystems Inc.
- Bio-Techne Corporation
- Biocare Medical, LLC
- Biorbyt Ltd.
- ENERGENESIS BIOMEDICAL CO., LTD.
- Enzo Biochem Inc.
- Geno Technology Inc.
- Kementec Solutions
- LI-COR Biotech, LLC
- Nepenthe
- Santa Cruz Biotechnology Inc.
- SurModics, Inc.
- Vector Laboratories, Inc.
- VWR International, LLC.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Protein-Free Blocking Buffer market report include:- Thermo Fisher Scientific Inc.
- Merck KGaA
- Danaher Corporation
- Bio-Rad Laboratories, Inc.
- PerkinElmer, Inc.
- Abcam plc
- Becton, Dickinson and Company
- Agilent Technologies, Inc.
- QIAGEN N.V.
- Takara Bio Inc.
- Advansta Inc.
- Avantor, Inc.
- Azure Biosystems Inc.
- Bio-Techne Corporation
- Biocare Medical, LLC
- Biorbyt Ltd.
- ENERGENESIS BIOMEDICAL CO., LTD.
- Enzo Biochem Inc.
- Geno Technology Inc.
- Kementec Solutions
- LI-COR Biotech, LLC
- Nepenthe
- Santa Cruz Biotechnology Inc.
- SurModics, Inc.
- Vector Laboratories, Inc.
- VWR International, LLC.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 191 |
| Published | October 2025 |
| Forecast Period | 2025 - 2032 |
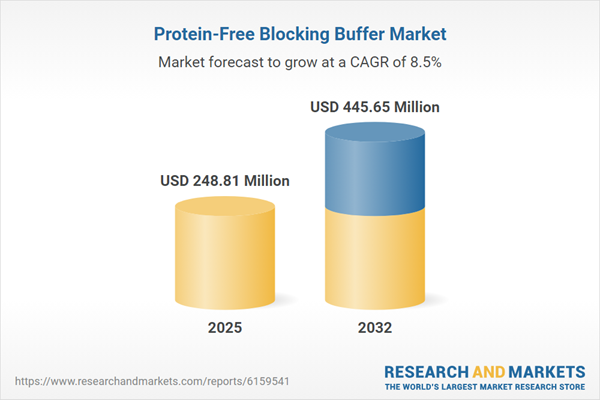
| Estimated Market Value ( USD | $ 248.81 Million |
| Forecasted Market Value ( USD | $ 445.65 Million |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |