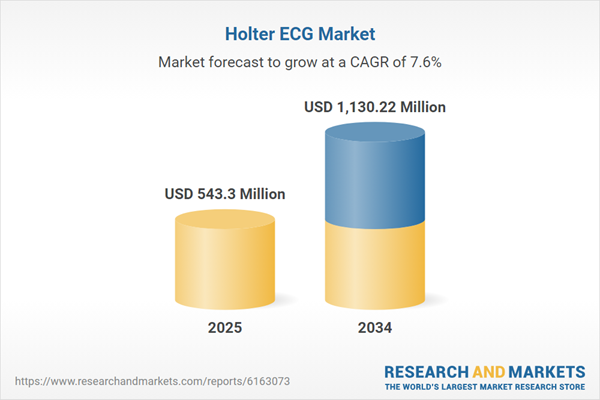
Global Holter ECG Market Overview
Holter ECG is an ambulatory electrocardiography device that records the heart rhythms of a patient for at least 24 hours. It is portable, consists of adhesive electrodes that are attached to the chest, and offers an accurate diagnosis of any cardiac condition over several days.The rising demand for 12-lead Holter ECG is driving the market growth of Holter ECG. The inclination towards 12-lead Holter ECG over traditional Holter ECG displaying two or three leads is due to the offering of vector information and its efficiency in diagnosing arrhythmias and localisation of myocardial ischemia, which is bolstering the market development of Holter ECG. In addition, they play a critical role in evaluating the effect of drugs or interventional therapeutic procedures in patients with heart failure and permanent atrial fibrillation, which is leading Holter ECG market growth.
Also, they offer information for risk stratification with the automatic analysis of parameters in 12-lead Holter ECG and are very accurate in the diagnosis of supraventricular tachycardia (SVT), ventricular tachycardia (VT), atrial flutter, atrial fibrillation, and monomorphic or Polymorphic VTs, thus invigorating the market growth. The efforts taken by market players in developing 12-lead Holter ECG with active electrodes, battery life, and that are compatible with T-shirts are likely to aid the market for Holter ECG over the forecast period.
The increasing prevalence of cardiovascular diseases and the mortality rates associated with them are contributing to the steady market growth of Holter ECG. The importance of Holter ECG in assisting doctors in diagnosing and providing accurate treatment for critical cardiac conditions by continuously monitoring the heart rhythms of a patient is providing impetus to market development. In addition, the rising preference for Holter ECG due to its portability and non-interference with the routine activities of the patients is contributing the Holter ECG market share. The reduced availability of medical staff is also contributing to the market expansion of personal healthcare systems such as Holter ECG.
Moreover, the Holter ECG plays a critical role in eliminating the need for extended stays at hospitals, offers better patient recovery in home environments, and is also cost-effective. The government initiatives in developing such long-term monitoring solutions for accurate diagnosis and reducing cardiovascular diseases among the population are propelling the market for Holter ECG. Further, the investments made in the development of Holter ECGs with extended recording and episode detection are expected to aid the Holter ECG market growth over the forecast period.
Prominent FDA Approvals
In May 2023, Abbott's Assert-IQ™ insertable cardiac monitor (ICM) received U.S. Food and Drug Administration (FDA) clearance, providing physicians with a new option for diagnostic evaluation and long-term monitoring of people experiencing irregular heartbeats. This clearance builds on Abbott's portfolio of connected health devices that can better help doctors manage and treat their patients remotely.ICM's small devices contain sensors that are inserted just under the skin of the chest. These devices are designed to monitor a person's heart constantly and in real time. Using Bluetooth® technology, Abbott's Assert-IQ ICM is designed to remain connected to a transmitter, usually the person's cell phone where it checks heart rhythms every 20 seconds, transmitting results in real-time to the clinic's portal.
The market for Holter ECG is expected to grow significantly with the development of a low-cost Holter monitor that is wireless and equipped with AI-assisted diagnostics. This innovative device promises quick and accurate cardiac screening, which plays a significant role in underprivileged areas where cardiovascular death rates are rising. By providing accessible and innovative healthcare solutions, this device has the potential to boost global Holter ECG market growth.
Additionally, in Jan 2024, iRhythm Technologies received European approval for its latest Zio wearable cardiac monitor, designed to detect irregular heartbeats such as atrial fibrillation. The new model is made compact, thinner, and lighter compared to the previous Zio XT ECG-recording patch. According to iRhythm, cases of arrhythmia and stroke have been on the rise in Europe, creating a sense of urgency for improved detection. The CE mark's green light covers both the 14-day Zio wearable monitor and its artificial intelligence-powered ECG analysis software, dubbed ZEUS.
The approval for the Zio wearable cardiac monitor in Europe which is also addressing the increasing cases of arrhythmia and stroke may result in Holter ECG market growth. The smaller design and advanced AI analysis offer improved detection, meeting the rising demand for enhanced cardiac monitoring solutions.
Research Activities Poised to Bring Innovation in the Market
October 2023, research conducted by Azlaan Ranjha, Laiba Jabbar, Osaid Ahmed focused on creating a wireless, transportable Holter monitor with a specific emphasis on improving cardiac disease diagnosis accuracy. The main goal was to develop a low-cost cardiac screening system tailored for underprivileged areas to address the increasing rates of cardiovascular death. The study demonstrated a deep neural network's superior diagnostic performance, surpassing cardiologist-level ECG analysis with over 88% accuracy. This breakthrough integrates wireless data transfer with cost-effective AI-assisted diagnostics, providing a swift and accurate cardiac screening option.The development of a wireless, low-cost Holter monitor with advanced AI-assisted diagnostics, achieving over 88% accuracy in cardiac anomaly identification may significantly boost the global Holter ECG market research by providing a more accessible and efficient cardiac screening solution, especially in underprivileged areas.
Global Holter ECG Market Segmentations
Holter ECG Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Lead Type
- Patch Type Single Monitor
- 3 Lead Holter Monitors
- 6 Lead Holter Monitors
- 12 Lead Holter Monitors
- Others
Market Breakup by Application
- Diagnostic
- Monitoring
Market Breakup by Product
- Wired Holter ECG Monitors
- Wireless Holter ECG Monitors
- Software
Market Breakup by End User
- Hospitals and Clinics
- Ambulatory Facilities
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Holter ECG Market Regional Analysis
North America is currently dominating the market due to factors such as the growing incidence of cardiovascular diseases, rising geriatric population, surging demand for remote monitoring, and the proliferation of advanced monitoring devices. The market is further propelled by technological advancements, preference shift towards ambulatory ECG monitoring, the introduction of next-generation wireless monitoring devices, and strategic technological collaborations among manufacturers.For instance, in May 2023, BIOTRONIK received U.S. Food and Drug Administration (FDA) approval for the BIOMONITOR IV implantable cardiac monitor (ICM), its latest innovation to improve the standard of care in cardiac monitoring. The device, featuring BIOTRONIK's advanced, AI-powered* SmartECG algorithm, will make its debut at the Heart Rhythm Society Congress 2023 in New Orleans.
BIOMONITOR IV, with SmartECG technology, significantly reduces false atrial fibrillation alerts by 86%, easing the burden on clinicians. It ensures 98% accuracy in detecting true episodes, helping timely and accurate diagnosis. This advancement is likely to boost the Holter ECG market technologies. In addition to the SmartECG technology, BIOMONITOR IV is the first ICM to discriminate between premature atrial contractions (PACs) and premature ventricular contractions (PVCs) marking a significant step forward in the domain of remote cardiac monitoring by providing healthcare professionals with reliable PAC and PVC trends for risk stratification and diagnosis.
The FDA approval for BIOMONITOR IV with advanced SmartECG technology significantly reduces false positive atrial fibrillation detections by 86%, aiding clinicians in accurate diagnoses. Its ability to differentiate between PACs and PVCs, along with enhanced remote monitoring features, is a major factor poised to positively influence the global Holter ECG market growth.
Global Holter ECG Market: Competitor Landscape
In May 2023, On May 2023, Philips revealed AI research predicting life-threatening ventricular arrhythmias at a Heart Rhythm Society congress. Using a deep neural network and 115,505 ECG recordings, the AI accurately forecasted sustained ventricular tachycardia over two weeks, showcasing potential in cardiac monitoring.The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
- GE Healthcare
- Philips Healthcare
- Medtronic plc
- Schiller AG
- Welch Allyn (now part of Hillrom)
- BioTelemetry, Inc. (now part of Philips)
- Spacelabs Healthcare (a subsidiary of OSI Systems, Inc.)
- Nihon Kohden Corporation
- ScottCare Corporation (a subsidiary of Biotronik)
- iRhythm Technologies, Inc.
- Cardionet (a subsidiary of BioTelemetry, Inc.)
- Applied Cardiac Systems, Inc.
- Compumed Inc.
- Scott Medical Products (a subsidiary of Cardinal Health)
- Midmark Corporation
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- GE Healthcare
- Philips Healthcare
- Medtronic plc
- Schiller AG
- Welch Allyn (now part of Hillrom)
- BioTelemetry, Inc. (now part of Philips)
- Spacelabs Healthcare (a subsidiary of OSI Systems, Inc.)
- Nihon Kohden Corporation
- ScottCare Corporation (a subsidiary of Biotronik)
- iRhythm Technologies, Inc.
- Cardionet (a subsidiary of BioTelemetry, Inc.)
- Applied Cardiac Systems, Inc.
- Compumed Inc.
- Scott Medical Products (a subsidiary of Cardinal Health)
- Midmark Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 543.3 Million |
| Forecasted Market Value ( USD | $ 1130.22 Million |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |