North America Glioma Market Analysis
Glioma is a tumor occurring in the brain or other locations in the nervous system such as the spinal cord and brain stem. Advancements in diagnostic technologies, including magnetic resonance imaging (MRI), positron emission tomography (PET), and molecular diagnostics, are contributing to the market expansion by increasing the detection rate, thus, driving the demand for glioma therapies. Further, the rising innovation in treatment modalities and the emphasis on personalized therapeutic approaches are expected to support the North America glioma market growth.Glioma is considered one of the most common forms of neoplasm in the central nervous system (CNS) originating from glial cells. It is estimated that 6 cases of gliomas are diagnosed per 100,000 individuals annually in the United States, accounting for 20,000 newly diagnosed patients with this primary brain tumor. In addition, gliomas occur most commonly in adults aged between 45 and 65 years. Recent data reveals that around 81.7% of all primary brain tumors are diagnosed in the adult population, with glioblastoma contributing to 16.4% of all primary brain tumor cases. The rising burden of this brain tumor along with the growing aging population more susceptible to developing the condition is poised to augment the North America glioma market demand in the forecast period.
The market value is influenced by the presence of a supportive regulatory framework that expedites the introduction of new treatments and diagnostic techniques. For instance, in February 2024, Servier Pharmaceuticals LLC's novel dual mIDH1/2 inhibitor vorasidenib (AG-881) new drug application was accepted and granted priority review by the United States Food and Drug Administration (FDA) for the treatment of patients with IDH-mutant diffuse glioma, a malignant and incurable brain tumor. The submission acceptance was prompted based on the positive findings of the phase 3 INDIGO trial. Moreover, to improve the diagnosis and management of glioma, the FDA granted fast-track designation to a PET imaging agent TLX101-CDx developed by Telix Pharmaceuticals Limited for use in progressive or recurrent glioma in April 2024 . Thus, the surge in approvals of advanced glioma therapies and diagnostic methods is anticipated to elevate the market value in the coming years.
North America Glioma Market Segmentation
The report offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- Astrocytoma
- Oligodendroglioma
- Glioblastoma
Market Breakup by Diagnosis
- Angiograms
- Magnetic Resonance Imaging (MRI)
- Computerized Tomography (CT)
- Surgical Biopsy
- Others
Market Breakup by Treatment Type
- Chemotherapy
- Radiation
- Surgery
Market Breakup by Route of Administration
- Oral
- Parenteral
Market Breakup by Country
- United States
- Canada
Leading Players in the North America Glioma Market
The key features of the market report include patent analysis, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:Pfizer Inc.
This leading research-based global biopharmaceutical company invests a substantial portion of its total R&D budget in the area of oncology and is actively involved in the clinical development of glioma drugs.Novartis AG
This Swiss multinational pharmaceutical company has a prominent presence in the market. Its FDA-approved Tafinlar + Mekinist is indicated for pediatric patients with BRAF V600E low-grade glioma.Johnson & Johnson
Johnson & Johnson, an American multinational, pharmaceutical, and medical technologies company, is engaged in merger and acquisition activities to expand its market presence. It is also involved in the research and development of innovative glioma treatments.Merck & Co., Inc.
Merck & Co., Inc., a leading science and technology company, leverages strategic collaborations to accelerate the market entry of cancer drug candidates and develops prescription products related to the treatment of brain tumors.Other players in the market include Roche Holding AG, Bristol-Myers Squibb Company, Amgen Inc., AstraZeneca PLC, Eli Lilly and Company, and AbbVie Inc.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Novartis AG
- Johnson & Johnson
- Merck & Co., Inc.
- Roche Holding AG
- Bristol-Myers Squibb Company
- Amgen Inc.
- AstraZeneca PLC
- Eli Lilly and Company
- AbbVie Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
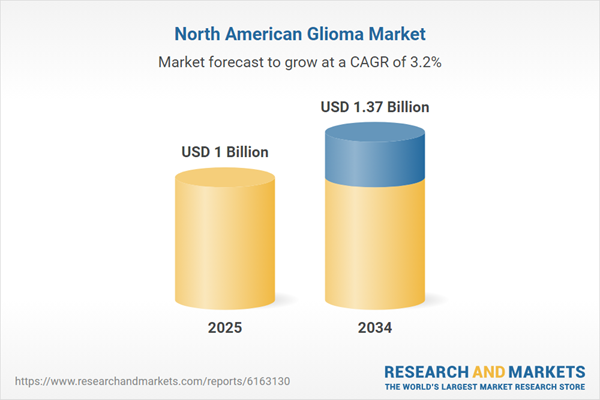
| Estimated Market Value ( USD | $ 1 Billion |
| Forecasted Market Value ( USD | $ 1.37 Billion |
| Compound Annual Growth Rate | 3.2% |
| Regions Covered | North America |
| No. of Companies Mentioned | 10 |