North America Hepatitis B Market Analysis
Hepatitis B is a vaccine-preventable liver infection that can result in a chronic infection leading to cirrhosis, liver cancer, and death. It is caused by the hepatitis B virus (HBV) and affects around 1.6 million people in the United States. The prevalence rate of the infection is higher in black, Hispanic, and Asian populations as compared to whites. Hepatitis B viral infection has the potential to progress to a chronic state and thus is a significant health challenge. Intensive research efforts are accelerating the development of innovative antiviral therapies and immune modulators, which is impacting the global hepatitis B market dynamics. Further, advancements in diagnostic technologies and improvements in healthcare infrastructure are some of the factors that contribute to the North America hepatitis B market growth.The market is influenced by the presence of a supportive regulatory environment that expedites the introduction of novel treatment options. In February 2024, the global biopharma company GSK plc announced that its investigational antisense oligonucleotide (ASO) bepirovirsen has received Fast Track Designation from the US Food and Drug Administration to treat chronic hepatitis B. Developed jointly with California-based biotechnology company Ionis Pharmaceuticals, the designation request was made based on the positive results from phase IIb trials B-Clear and B-Sure. The company claims that Bepirovirsen (in combination with oral nucleoside/nucleotide analogues) is the only single agent in phase III development that demonstrated a clinically meaningful functional cure response in chronic hepatitis B patients. Such substantial investment in clinical studies by the leading pharmaceutical companies to develop effective medications for this viral infection is poised to boost the North America hepatitis B market demand in the forecast period.
Increased awareness initiatives and education campaigns regarding hepatitis B are promoting early diagnosis and encouraging more people to get vaccinated and seek medical care. In the United States, the month of May is recognized as Hepatitis Awareness Month, and May 19th is designated as Hepatitis Testing Day. During this month, the national public health agency Centers for Disease Control and Prevention (CDC) in collaboration with its public health partners highlights the impact of this disease and raises awareness about viral hepatitis while emphasizing the need for testing and vaccination. Such campaigns also help to improve the understanding of viral hepatitis transmission, associated risk factors, and available treatment options, which is expected to elevate the market value.
North America Hepatitis B Market Segmentation
The report offers a detailed analysis of the market based on the following segments:Market Breakup by Type:
- Acute
- Chronic
Market Breakup by Treatment:
- Immune Modulator Drugs
- Antiviral Drugs
- Hepatoprotectives
- Vaccine
- Surgery
Market Breakup by Route of Administration:
- Oral
- Parenteral
- Others
Market Breakup by Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Market Breakup by Region:
- United States of America
- Canada
Leading Companies in the North America Hepatitis B Market
The key features of the market report include funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:Gilead Sciences, Inc.
This American biopharmaceutical received FDA approval for its drug Vemlidy® (tenofovir alafenamide) for the treatment of chronic hepatitis B virus infection in pediatric patients in 2022.Merck & Co., Inc.
Merck & Co., Inc. is a leading science and technology company with a robust market presence. Its vaccine RECOMBIVAX HB is taken to prevent infection from all known subtypes of the hepatitis B virus.GlaxoSmithKline plc (GSK)
This global provider of innovative vaccines and specialty medicines is actively engaged in strategic partnerships to develop pharmaceutical products. Its investigational antisense oligonucleotide is currently undergoing a phase III trial to assess its efficacy for the treatment of chronic hepatitis B.
Novartis AG
Novartis AG, a global healthcare company based in Switzerland, is committed to the development of treatment solutions for hepatitis B and C infections.Other players in the market include Pfizer Inc., Sanofi, Johnson & Johnson, AbbVie Inc., Bristol Myers Squibb, and Dynavax Technologies.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Gilead Sciences, Inc.
- Merck & Co., Inc.
- GlaxoSmithKline plc (GSK)
- Novartis AG
- Pfizer Inc.
- Sanofi
- Johnson & Johnson
- AbbVie Inc.
- Bristol Myers Squibb
- Dynavax Technologies
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
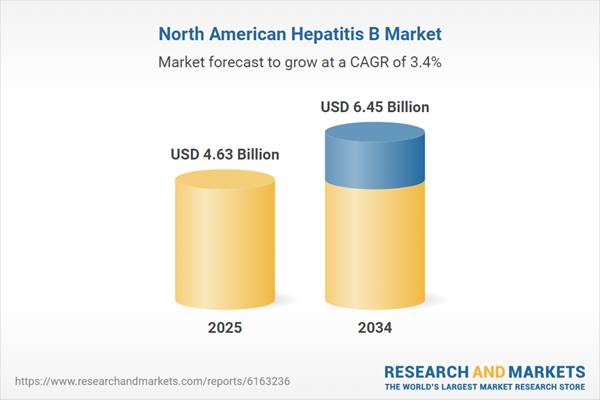
| Estimated Market Value ( USD | $ 4.63 Billion |
| Forecasted Market Value ( USD | $ 6.45 Billion |
| Compound Annual Growth Rate | 3.4% |
| Regions Covered | North America |
| No. of Companies Mentioned | 10 |