Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
The market is poised for sustained expansion, supported by national priorities around early diagnosis, rapid treatment pathways, and clinical outcomes optimization. Strategic growth opportunities are emerging in rural healthcare integration, upskilling of interventional specialists, and the domestic scaling of device manufacturing and distribution networks aimed at improving both accessibility and cost-efficiency across Australia's geographically dispersed healthcare system.
Key Market Drivers
Rising Incidence of Stroke and Neurovascular Disorders
The rising incidence of stroke and neurovascular disorders is one of the most significant drivers accelerating the growth of the Australia Neurovascular Devices Market, as it directly expands the addressable patient population and increases the clinical demand for advanced interventional solutions. Stroke is the second leading cause of death and a leading cause of disability in Australia. According to data from the Stroke Foundation - Australia, approximately 27,400 Australians suffer a stroke each year, underscoring the country’s growing cerebrovascular disease burden. Looking ahead, the Foundation projects a more than 50% increase in annual stroke incidence by 2050, with the number of new cases expected to reach around 50,600 per year.This sharp upward trajectory reflects broader demographic shifts, including an aging population and rising prevalence of comorbid risk factors, and is poised to significantly drive demand for advanced neurovascular interventions and device-based therapies across the Australian healthcare system. The surge in stroke cases is leading to increased hospital admissions, thereby creating consistent demand for neurovascular devices such as stent retrievers, aspiration catheters, guide catheters, and embolization coils. Hospitals and stroke centers are compelled to expand their inventory and capabilities to manage higher caseloads efficiently, resulting in greater device utilization and repeat purchases.
Between 2019 and 2038, it is projected that 644,208 Australians will experience a first-time ischemic stroke, encompassing both fatal and nonfatal cases. This figure highlights the substantial and growing clinical burden of ischemic stroke in Australia, signaling a sustained demand for advanced diagnostic and interventional neurovascular technologies over the coming decades.
A significant portion of strokes in Australia are ischemic (caused by a blockage of blood flow to the brain), which are now increasingly treated with mechanical thrombectomy. This minimally invasive procedure requires a range of specialized devices to restore cerebral blood flow quickly and safely. The shift toward mechanical thrombectomy has created a new standard of care, encouraging hospitals to invest in neurovascular product portfolios. As more patients become eligible for thrombectomy thanks to improved stroke awareness and faster imaging there is an associated increase in procedural volumes, which fuels demand for consumable neurovascular devices.
Neurological disorders represent a substantial public health challenge in Australia, with 43% of the population equivalent to approximately 10.6 million individuals diagnosed with a neurological condition. These disorders are not only widespread but also account for a disproportionate share of the national disease burden, contributing to over 20%. This escalating prevalence underscores the urgent need for expanded neurovascular care infrastructure and advanced therapeutic solutions within the Australian healthcare system.
Beyond stroke, the prevalence of cerebral aneurysms, arteriovenous malformations (AVMs), and intracranial atherosclerosis is also increasing in Australia. Many of these conditions require interventional treatment with devices such as flow diverters, detachable coils, and balloon catheters. These conditions often require follow-up treatments or monitoring procedures, contributing to sustained demand for neurovascular device usage. The broadening scope of neurovascular care, from emergency interventions to elective repairs, is expanding revenue opportunities across both public and private hospital systems.
Key Market Challenges
Limited Access to Advanced Neurointervention in Regional and Remote Areas
Australia's vast geography poses significant healthcare delivery barriers, particularly outside metropolitan hubs such as Sydney, Melbourne, and Brisbane. Advanced neurovascular procedures such as mechanical thrombectomy or aneurysm coiling require specialized equipment, trained personnel, and high-end imaging infrastructure, which are often lacking in regional and rural hospitals.Underutilization of neurovascular devices in non-urban areas despite patient need. Delayed treatment for stroke and cerebral emergencies, narrowing the window of opportunity for device-based interventions. Reduced commercial ROI for manufacturers targeting the national market without scalable rural access strategies. To unlock growth, device manufacturers and healthcare policymakers must co-develop models for remote intervention support such as mobile stroke units, AI-driven triage platforms, and cross-hospital device sharing programs.
Key Market Trends
Integration of Artificial Intelligence and Advanced Neuroimaging
The deployment of AI-driven imaging platforms is revolutionizing acute stroke diagnosis and treatment planning. Tools such as RAPID.AI and Viz.ai are increasingly being integrated into emergency workflows to detect ischemia, hemorrhage, and perfusion deficits in real time.Enhances diagnostic speed and accuracy, enabling faster decision-making for device-based interventions. Supports rural and remote stroke networks, where real-time teleradiology is critical for patient triage and transfer decisions. Facilitates clinical trial enrollment and post-procedural monitoring, increasing hospital uptake of neurovascular technologies. Vendors with AI-compatible devices or partnerships with imaging software firms will gain competitive advantage through better clinical integration and physician adoption.
Key Market Players
- Stryker
- Integra LifeSciences Corporation
- Terumo Australia Pty Limited
- MicroPort CRM
- Penumbra, Inc.
- Acandis GmbH
- Medtronic
Report Scope:
In this report, the Australia Neurovascular Devices Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Australia Neurovascular Devices Market, By Device:
- Cerebral Embolization and Aneurysm Coiling Devices
- Cerebral Angioplasty and Stenting Systems
- Neurothrombectomy Devices
- Support Devices
- Trans Radial Access Devices
Australia Neurovascular Devices Market, By Therapeutic Application:
- Stroke
- Cerebral Artery
- Cerebral Aneurysm
- Others
Australia Neurovascular Devices Market, By End User:
- Hospitals
- Specialty Clinics
- Others
Australia Neurovascular Devices Market, By Region:
- Northern Vietnam
- Central Vietnam
- Southern Vietnam
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Australia Neurovascular Devices Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Stryker
- Integra LifeSciences Corporation
- Terumo Australia Pty Limited
- MicroPort CRM
- Penumbra, Inc.
- Acandis GmbH
- Medtronic
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 85 |
| Published | August 2025 |
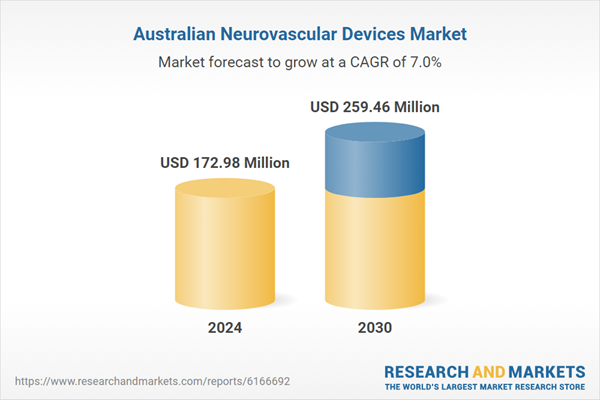
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 172.98 Million |
| Forecasted Market Value ( USD | $ 259.46 Million |
| Compound Annual Growth Rate | 6.9% |
| Regions Covered | Australia |
| No. of Companies Mentioned | 7 |