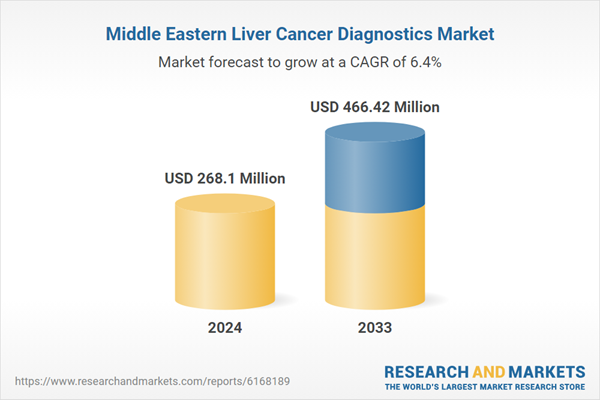
The Middle East liver cancer diagnostics market is expanding rapidly, driven by epidemiological trends, strategic alliances, and accelerated adoption of advanced technologies. Hepatocellular carcinoma (HCC), the most common form of liver cancer, continues to rise across the region, with different etiological patterns shaping country-level dynamics. Egypt has long borne the burden of hepatitis C-driven liver cancer, while Gulf states such as Saudi Arabia, the UAE, Kuwait, and Qatar are witnessing increasing cases linked to obesity, diabetes, and metabolic syndrome. This epidemiological transition underscores the pressing need for sensitive, non-invasive diagnostic tools, supported by public health initiatives and stronger cancer registries. Despite progress in viral hepatitis management, lifestyle-related drivers continue to fuel incidence, requiring diagnostics that can enable earlier detection and intervention.
The competitive landscape is being reshaped by global biotech firms and regional healthcare leaders. In October, 2024, Helio Genomics entered a strategic partnership with Halub Medical Co. to commercialize HelioLiver Dx in Saudi Arabia and the broader GCC. The test, expected to be launched commercially in Q1 2025, is projected to generate more than USD 10 million in revenue in its first year of availability. HelioLiver Dx leverages AI-driven multi-analyte technology-analyzing cfDNA, methylation signatures, serum proteins, and demographic markers and demonstrated four-times higher sensitivity than ultrasound in pivotal U.S. trials led by Stanford Medical Centers. Importantly, it offers adherence rates nearly nine-times greater than conventional surveillance, addressing a critical gap in patient compliance across the Middle East. The partnership aligns with Saudi Arabia’s Health Cluster initiatives and Vision 2030 strategy, reflecting how international innovation is being localized to address regional healthcare burdens.
Middle East Liver Cancer Diagnostics Market Report Segmentation
This report forecasts revenue growth at regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. The analyst has segmented the Middle East liver cancer diagnostics market report on the basis of test type, end-use, country:Middle East Liver Cancer Diagnostics Test type Outlook (Revenue, USD Million, 2021-2033)
- Laboratory Tests
- Biomarkers
- Oncofetal and Glycoprotein Antigens
- Enzymes and Isoenzymes
- Growth Factors and Receptors
- Molecular Markers
- Pathological Biomarkers
- Blood Tests
- Imaging
- Endoscopy
- Biopsy
Middle East Liver Cancer Diagnostics End-use Outlook (Revenue, USD Million, 2021-2033)
- Hospitals & Diagnostic Laboratories
- Academic & Research Institutes
- Pharmaceutical & CRO Laboratories
- Country Outlook (Revenue in USD Million, 2021-2033)
- Middle East
- Saudi Arabia
- UAE
- Kuwait
- Qatar
- Oman
- Rest of MEA
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the industry across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segmental and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listings for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The major companies profiled in this Middle East Liver Cancer Diagnostics market report include:- Abbott Laboratories
- Thermo Fisher Scientific, Inc.
- F. Hoffmann-La Roche Ltd.
- Qiagen N.V.
- Siemens Healthineers
- Becton, Dickinson & Company
- Illumina, Inc.
- Epigenomics AG
- Koninklijke Philips N.V.
- Fujifilm Medical Systems U.S.A., Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | August 2025 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 268.1 Million |
| Forecasted Market Value ( USD | $ 466.42 Million |
| Compound Annual Growth Rate | 6.3% |
| Regions Covered | Middle East |
| No. of Companies Mentioned | 11 |