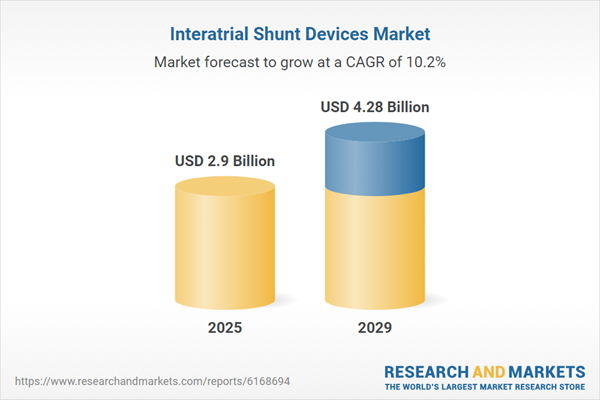
The interatrial shunt devices market size is expected to see rapid growth in the next few years. It will grow to $4.28 billion in 2029 at a compound annual growth rate (CAGR) of 10.2%. The expected growth in the forecast period can be linked to the increasing prevalence of heart failure, an expanding elderly population, heightened awareness of minimally invasive cardiac procedures, rising healthcare spending, and greater adoption of catheter-based treatments. Key trends during this period include innovations in shunt device materials, technology-driven miniaturization of interventional devices, integration of real-time imaging and navigation technologies, advancements in catheter-based delivery methods, and improvements in hemodynamic monitoring systems.
The increasing prevalence of heart failure is expected to drive the growth of the interatrial shunt devices market in the coming years. Heart failure is a condition where the heart cannot pump blood effectively, leading to insufficient delivery of oxygen and nutrients to the body’s tissues. The rise in heart failure cases is largely due to the growing incidence of hypertension, which gradually weakens the heart’s pumping ability. Interatrial shunt devices assist in managing heart failure by creating a small opening between the left and right atria, allowing excess pressure from the overloaded left atrium to be relieved by transferring it to the right atrium. This helps reduce pulmonary congestion and alleviates symptoms. For example, in September 2024, the Heart Failure Society of America, a US-based professional organization, reported that approximately 6.7 million Americans aged 20 and older were living with heart failure in 2024. This figure is expected to rise to 11.4 million by 2050. Consequently, the growing prevalence of heart failure is propelling the expansion of the interatrial shunt devices market.
Key players in the interatrial shunt devices market are concentrating on innovative developments, such as first-in-human (FIH) clinical trials to assess the safety and effectiveness of new shunt technologies aimed at lowering left atrial pressure in heart failure patients. A first-in-human clinical trial is the initial phase of testing a new medical device or drug in humans to evaluate safety, tolerability, and early efficacy following successful preclinical research. For instance, in January 2025, Adona Medical Inc., a US-based medical technology company, completed enrollment for its first-in-human (FIH) clinical trial, ATHENS-HF, involving a novel interatrial shunt designed for heart failure treatment. This device is among the first to combine therapeutic shunting with integrated bi-atrial pressure monitoring, offering a more adaptive and personalized method of managing heart failure.
In October 2024, Johnson & Johnson, a US-based pharmaceutical company, acquired V-Wave Ltd. for an undisclosed sum. This acquisition aims to strengthen Johnson & Johnson’s cardiovascular portfolio by adding advanced heart failure therapies and addressing critical unmet needs in the sector. V-Wave Ltd. is an Israel-based medical device firm specializing in innovative interatrial shunt devices.
Major players in the interatrial shunt devices market are Johnson & Johnson, Abbott Laboratories, Medtronic plc., Terumo Corporation, Edwards Lifesciences Corporation, Cook Medical, Integra LifeSciences Holdings Corporation, Lepu Medical Technology (Beijing) Co. Ltd., B. Braun Melsungen AG., W. L. Gore & Associates Inc., Venus Medtech Inc., Occlutech Holding AG., Wuhan Vickor Medical Technology Co. Ltd., Corvia Medical Inc., Xeltis BV, Alleviant Medical Inc., Cerevasc Inc., Adona Medical Inc., Carag AG., InterShunt Technologies Inc.
North America was the largest region in the interatrial shunt devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in interatrial shunt devices report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the interatrial shunt devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The interatrial shunt devices market consists of sales of catheter-based delivery tools, transseptal access kits, monitoring accessories, and procedure-specific consumables. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The fast surge in U.S. tariffs and the trade tensions that followed in spring 2025 are heavily affecting the medical equipment sector, particularly for imported imaging machine components, surgical-grade stainless steel, and plastic disposables. Hospitals and clinics resist price hikes, pressuring manufacturers’ margins. Regulatory hurdles compound the problem, as tariff-related supplier changes often require re-certification of devices, delaying time-to-market. Companies are mitigating risks by dual-sourcing critical parts, expanding domestic production of commoditized items, and accelerating R&D in cost-efficient materials.
The interatrial shunt devices market research report is one of a series of new reports that provides interatrial shunt devices market statistics, including the interatrial shunt devices industry's global market size, regional shares, competitors with the interatrial shunt devices market share, detailed interatrial shunt devices market segments, market trends and opportunities, and any further data you may need to thrive in the interatrial shunt devices market. This interatrial shunt devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Interatrial shunt devices are implantable medical tools developed to create a controlled passage between the left and right atria of the heart, aiming to lower pressure and alleviate symptoms in heart failure patients. These devices help redirect blood flow from the left atrium to the right atrium, effectively reducing elevated left atrial pressure associated with heart failure.
The primary types of interatrial shunt devices include implantable shunt devices and temporary shunt devices. Implantable shunt devices are designed to create a regulated connection between different body compartments and are typically used to redirect fluid or pressure for therapeutic purposes. These devices can be placed via surgical procedures or transcatheter methods. Common construction materials include nitinol, polyester, and polypropylene. They are primarily used in the treatment of congenital heart defects, heart failure, and stroke prevention, and are employed across various healthcare settings such as hospitals, ambulatory surgical centers, cardiac catheterization labs, and others.
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Interatrial Shunt Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on interatrial shunt devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for interatrial shunt devices? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The interatrial shunt devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Device Type: Implantable Shunt Devices; Temporary Shunt Devices2) By Delivery Method: Surgical Delivery; Transcatheter Delivery
3) By Material: Nitinol; Polyester; Polyproplene
4) By Application: Congenital Heart Disease; Heart Failure; Stroke Prevention
5) By End-User: Hospitals; Ambulatory Surgical Centers; Cardiac Catheterization Laboratories; Other Users
Subsegments:
1) By Implantable Shunt Devices: Single-Lumen Shunt Devices; Expandable Shunt Devices; Multi-Lumen Shunt Devices2) By Temporary Shunt Devices: Balloon-Assisted Shunt Devices; Percutaneous Temporary Shunt Devices; Catheter-Based Shunt Devices
Companies Mentioned: Johnson & Johnson; Abbott Laboratories; Medtronic plc.; Terumo Corporation; Edwards Lifesciences Corporation; Cook Medical; Integra LifeSciences Holdings Corporation; Lepu Medical Technology (Beijing) Co. Ltd.; B. Braun Melsungen AG.; W. L. Gore & Associates Inc.; Venus Medtech Inc.; Occlutech Holding AG.; Wuhan Vickor Medical Technology Co. Ltd.; Corvia Medical Inc.; Xeltis BV; Alleviant Medical Inc.; Cerevasc Inc.; Adona Medical Inc.; Carag AG.; InterShunt Technologies Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain.
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Interatrial Shunt Devices market report include:- Johnson & Johnson
- Abbott Laboratories
- Medtronic plc.
- Terumo Corporation
- Edwards Lifesciences Corporation
- Cook Medical
- Integra LifeSciences Holdings Corporation
- Lepu Medical Technology (Beijing) Co. Ltd.
- B. Braun Melsungen AG.
- W. L. Gore & Associates Inc.
- Venus Medtech Inc.
- Occlutech Holding AG.
- Wuhan Vickor Medical Technology Co. Ltd.
- Corvia Medical Inc.
- Xeltis BV
- Alleviant Medical Inc.
- Cerevasc Inc.
- Adona Medical Inc.
- Carag AG.
- InterShunt Technologies Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.9 Billion |
| Forecasted Market Value ( USD | $ 4.28 Billion |
| Compound Annual Growth Rate | 10.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |