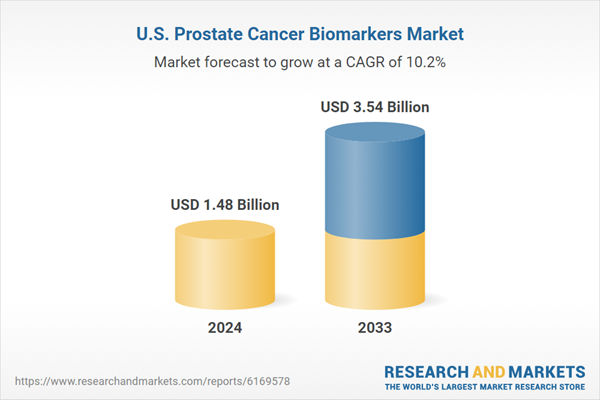
United States Prostate Cancer Biomarkers Market is expected to reach US$ 3.54 billion by 2033 from US$ 1.48 billion in 2024, with a CAGR of 10.16% from 2025 to 2033. The market for prostate cancer biomarkers in the US is expected to develop due to factors such rising prostate cancer prevalence, improved diagnostic technology, and an increased emphasis on customized therapy for early detection and treatment.
United States Prostate Cancer Biomarkers Industry Overview
The market for prostate cancer biomarkers in the US has become a crucial area of oncology due to the rising incidence of prostate cancer and the urgent need for precise diagnostic and prognostic instruments. Advances in biomarkers have been made possible by the limitations of traditional techniques, such as PSA testing, which has been plagued by false positives and a lack of specificity. Nowadays, determining illness risk, directing treatment plans, and tracking therapeutic results all depend heavily on biomarkers. The increasing focus on personalized medicine is making these solutions essential to raising patient survival rates and treatment quality.One of the most common cancers that affect males in the US is prostate cancer. One in eight males may receive a cancer diagnosis at some point in their lives, according to the American Cancer Society. It is the second biggest cause of cancer death among American men, with an anticipated 268,500 new cases and 34,500 fatalities reported in 2022 alone. Prostate cancer affects more than 3.1 million men in the United States today. The need for novel biomarkers that can facilitate earlier detection, better risk stratification, and treatment personalization has increased due to this sizable patient population and limitations in conventional diagnostics like PSA testing, which lacks the specificity to differentiate between benign and malignant conditions.
During the American Urological Association's (AUA) 2025 Annual Meeting on April 28, 2025, OncoAssure, a medical technology business based in Ireland, introduced the OncoAssure Prostate Test in the United States. Designed for individuals with localized prostate cancer, this next-generation prognostic test can be done either after surgery or after a biopsy. The test provides an accurate assessment of disease aggressiveness and recurrence risk by combining genomic analysis of "Master Driver" genes with clinical characteristics, such as the CAPRA score. This method reduces needless overtreatment by assisting clinicians in differentiating between aggressive and indolent tumors.
Diacarta Inc., a partner of OncoAssure, will carry out testing in its Pleasanton, California, ISO and CAP/CLIA-certified facility. A 2025 financing round is planned by the business to assist pipeline extension and commercialization, including the creation of a melanoma prognostic test.
Key Factors Driving the United States Prostate Cancer Biomarkers Market Growth
Rising Prevalence of Prostate Cancer
The increasing incidence of prostate cancer in the United States has heightened demand for effective diagnostic and prognostic tools. Traditional PSA testing often produces false positives, leading to unnecessary biopsies and patient anxiety. Advanced biomarkers, however, provide more specific and sensitive detection, helping differentiate aggressive cancers from slow-growing forms. This accuracy allows clinicians to tailor treatment strategies, improving patient outcomes while reducing overtreatment. The rising disease burden also emphasizes the importance of early detection, where biomarker-based tests play a crucial role. With prostate cancer ranking among the most diagnosed cancers in men, the growing need for reliable, personalized diagnostic solutions is a key driver for the expansion of the biomarker market across the country.Advancements in Molecular and Genomic Technologies
Technological progress in genomics, proteomics, and molecular diagnostics has significantly enhanced biomarker discovery and application in prostate cancer. Sophisticated platforms now allow clinicians to analyze genetic mutations, protein expressions, and metabolic signatures associated with the disease. These advancements enable earlier detection, risk stratification, and monitoring of treatment responses, creating value for both patients and healthcare providers. Liquid biopsy technologies, which use blood or urine samples, are gaining traction as non-invasive alternatives to traditional tissue biopsies. Their ability to provide real-time insights into disease progression is transforming clinical decision-making. As research funding and commercial investment continue to drive innovation, advanced technologies will remain a cornerstone in expanding the adoption and clinical integration of prostate cancer biomarkers in the U.S.Growing Focus on Precision Medicine and Personalized Care
The increasing shift toward precision medicine is a major driver for the prostate cancer biomarkers market in the U.S. Biomarker-based tests allow for personalized treatment strategies tailored to individual patient profiles, genetic predispositions, and tumor characteristics. This precision reduces the risk of ineffective treatments and enhances patient survival outcomes. Clinical guidelines are progressively incorporating biomarker-driven diagnostics, reinforcing their role in decision-making. Pharmaceutical companies are also leveraging biomarkers in drug development, using them for patient stratification and monitoring therapeutic efficacy in clinical trials. With healthcare providers and policymakers emphasizing personalized care, biomarker integration is becoming a standard approach. This growing adoption underscores the importance of biomarkers in advancing both clinical practice and patient-centered oncology care.Challenges in the United States Prostate Cancer Biomarkers Market
High Costs and Reimbursement Barriers
One of the key challenges in the U.S. prostate cancer biomarkers market is the high cost of advanced diagnostic tests. While biomarker technologies offer improved accuracy and clinical value, their associated expenses often make them less accessible to patients without comprehensive insurance coverage. Reimbursement policies can vary, with some tests not fully covered, creating financial burdens for patients and limiting adoption among healthcare providers. Smaller clinics and community hospitals may also struggle with implementation due to the cost of specialized equipment and training. Unless affordability and reimbursement frameworks are streamlined, these financial hurdles could slow the widespread adoption of biomarker-based testing, preventing equitable access across diverse patient populations in the United States.Regulatory Complexity and Clinical Validation
Another challenge is navigating the regulatory environment and achieving sufficient clinical validation for new biomarker tests. Regulatory authorities require extensive evidence to confirm the clinical utility, safety, and accuracy of these diagnostics, often resulting in lengthy approval timelines. Many biomarkers remain in the research phase due to challenges in demonstrating consistent outcomes across diverse patient populations. Clinical adoption can also be hindered by hesitancy among providers who prefer established diagnostic tools until new biomarkers prove reliability in real-world settings. Furthermore, aligning biomarker tests with evolving clinical guidelines requires ongoing validation studies and collaborations. This regulatory and clinical complexity slows market entry for innovative solutions, limiting their immediate availability and broader impact in prostate cancer care.United States Prostate Cancer Biomarkers Market Overview by States
Prostate cancer biomarker adoption varies by region in the United States, but it is particularly high in California, Texas, New York, and Florida, where the market is growing due to sophisticated healthcare infrastructure, research centers, and awareness campaigns. The following provides a market overview by States:
California Prostate Cancer Biomarkers Market
California represents a leading market for prostate cancer biomarkers due to its robust healthcare ecosystem and advanced research infrastructure. The state’s strong presence of biotechnology firms, academic institutions, and oncology centers has fueled innovation and clinical adoption of biomarker-based diagnostics. Public health initiatives and awareness programs actively promote early detection, increasing testing rates among at-risk populations. Urban hubs like Los Angeles, San Francisco, and San Diego drive adoption through cutting-edge clinical practices, while community outreach programs aim to expand access in underserved areas. California’s diverse population also contributes to the need for personalized care, further supporting biomarker use. With a strong focus on precision medicine, the state continues to play a pivotal role in advancing biomarker-driven cancer care in the U.S.Texas Prostate Cancer Biomarkers Market
Texas holds a significant position in the U.S. prostate cancer biomarkers market, supported by its expansive healthcare network and growing research collaborations. Major urban centers like Houston and Dallas feature advanced oncology institutions and clinical trial activity that promote biomarker adoption. The state has prioritized early cancer detection through awareness campaigns and public health initiatives, increasing the demand for more accurate diagnostic tools. However, disparities between urban and rural healthcare access remain a challenge. Efforts to expand testing availability in underserved areas are being supported through partnerships between healthcare providers and research organizations. With continued investments in personalized medicine and a focus on patient-centered care, Texas remains a vital contributor to the growth of the prostate cancer biomarker market in the country.New York Prostate Cancer Biomarkers Market
New York is a hub for prostate cancer biomarker adoption, driven by its advanced healthcare infrastructure and concentration of academic research institutions. The state actively promotes innovation in oncology through clinical trials, public health initiatives, and private sector investments. New York City, in particular, leads with widespread availability of advanced biomarker testing in hospitals and diagnostic laboratories. Awareness campaigns and insurance coverage support encourage broader adoption, while targeted initiatives address access disparities among minority and underserved populations. The integration of biomarkers into clinical practice aligns with the state’s focus on precision medicine and personalized healthcare delivery. With its combination of cutting-edge research, robust healthcare infrastructure, and emphasis on prevention, New York continues to play a critical role in shaping the U.S. biomarker landscape.Florida Prostate Cancer Biomarkers Market
Florida is a key state in the U.S. prostate cancer biomarkers market, with its large elderly population contributing to rising demand for accurate diagnostic tools. The state’s healthcare system is increasingly adopting biomarker-based diagnostics for early detection and personalized treatment planning. Urban centers such as Miami, Tampa, and Orlando are leading adoption, supported by advanced clinical practices and access to molecular diagnostic technologies. Public health programs and educational initiatives are raising awareness of prostate cancer prevention and screening, further driving demand. However, disparities in access remain in rural regions, requiring focused outreach. With its emphasis on early detection and preventive healthcare, Florida is poised to remain a strong market for prostate cancer biomarkers, significantly contributing to the overall U.S. market growth.Recent Developments in U.S. Prostate Cancer Biomarkers Market
- The AI-driven prostate cancer technology platform PATHOMIQ_PRAD was licensed exclusively in the United States by Myriad Genetics and PATHOMIQ in February 2025. Through this collaboration, Myriad's oncology portfolio will incorporate AI-enabled diagnostics, facilitating better treatment choices both prior to and following prostate cancer treatment. In line with changing demands in the market for prostate cancer biomarkers, the partnership seeks to improve diagnostic accuracy in prostate cancer treatment by utilizing cutting-edge artificial intelligence.
- DiaCarta and OncoAssure Ltd. signed a strategic partnership in February 2024 to market a test for prostate cancer prognosis. This six-gene expression assay calculates the likelihood of a biochemical recurrence within five years after surgery and assesses the risk of aggressive illness after diagnosis. Through biomarker-based risk stratification, the collaboration supports the test's validation and market expansion by leveraging DiaCarta's clinical diagnostic capabilities, which helps to provide more individualized prostate cancer treatment.
Market Segmentations
Type
- Genetic Biomarker
- Cell-based Biomarkers
- Metabolomic Biomarkers
Application
- Screening And Early Detection
- Diagnostic And Risk Stratification
- Prognosis And Treatment Monitoring
- Companion Diagnostics
End Use
- Hospitals & Diagnostic Laboratories
- Academic & Research Institutes
- Biopharmaceutical Companies
States
- California
- Texas
- New York
- Florida
- Illinois
- Pennsylvania
- Ohio
- Georgia
- New Jersey
- Washington
- North Carolina
- Massachusetts
- Virginia
- Michigan
- Maryland
- Colorado
- Tennessee
- Indiana
- Arizona
- Minnesota
- Wisconsin
- Missouri
- Connecticut
- South Carolina
- Oregon
- Louisiana
- Alabama
- Kentucky
- Rest of United States
All the Key players have been covered
- Overviews
- Key Person
- Recent Developments
- SWOT Analysis
- Revenue Analysis
Company Analysis:
- Exact Sciences Corp
- Myriad Genetics Inc
- BIO-TECHNE Corp
- OPKO HEALTH,INC.
- MDxHealth SA
- Veracyte Inc
- Roche Diagnostic Ltd.
- Pfizer Inc.
Table of Contents
Companies Mentioned
- Exact Sciences Corp
- Myriad Genetics Inc
- BIO-TECHNE Corp
- OPKO HEALTH,INC.
- MDxHealth SA
- Veracyte Inc
- Roche Diagnostic Ltd.
- Pfizer Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | August 2025 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 1.48 Billion |
| Forecasted Market Value ( USD | $ 3.54 Billion |
| Compound Annual Growth Rate | 10.1% |
| Regions Covered | United States |
| No. of Companies Mentioned | 8 |