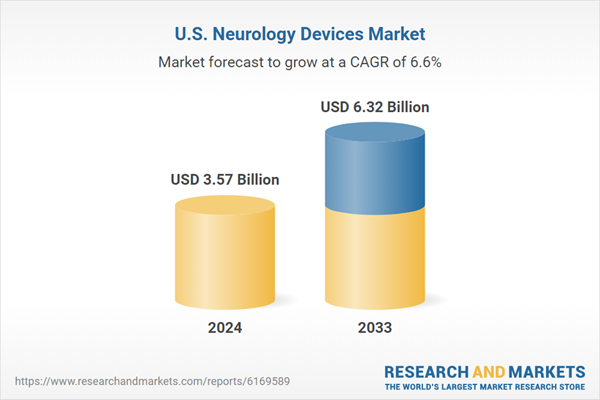
United States Neurology Devices Market is expected to reach US$ 6.32 billion by 2033 from US$ 3.57 billion in 2024, with a CAGR of 6.57% from 2025 to 2033. The market is anticipated to expand gradually due to advancements in technology, an increase in neurological conditions, and a growing need for sophisticated diagnostic and treatment options in healthcare institutions.
United States Neurology Devices Industry Overview
The United States neurology devices industry is experiencing robust growth, fueled by rising incidences of neurological disorders such as Alzheimer’s disease, Parkinson’s disease, epilepsy, and stroke. These conditions are creating strong demand for advanced diagnostic, monitoring, and therapeutic devices. Neurology devices play a vital role in early detection, effective treatment, and improved patient management. Technologies such as neurostimulation, neurosurgical devices, and neurodiagnostic tools are gaining significant traction in clinical settings. Hospitals and specialized clinics across the country are increasingly adopting innovative systems to improve outcomes and reduce the burden of neurological conditions. This growing healthcare demand highlights the essential role of neurology devices in addressing both acute and chronic neurological challenges.Innovation is a central driver in shaping the competitive landscape of the neurology devices industry. Breakthroughs in minimally invasive neurosurgical techniques, wearable neurodiagnostic tools, and brain-computer interface technologies are expanding the capabilities of these devices. Companies are heavily investing in research and development to enhance device performance, safety, and user-friendliness. Partnerships between medical device manufacturers, research institutions, and healthcare providers are further accelerating the introduction of next-generation technologies. Additionally, artificial intelligence (AI) and digital health solutions are increasingly being integrated with neurology devices, enabling real-time monitoring, predictive analytics, and personalized treatment approaches for neurological patients.
Despite promising advancements, the industry faces several challenges, including high device costs, reimbursement complexities, and regulatory hurdles. Many neurology devices require extensive clinical validation before approval, slowing market entry. Smaller medical device companies often struggle with these barriers, limiting competitive diversity. Nevertheless, the overall outlook for the U.S. neurology devices market remains highly positive. Continuous innovation, rising healthcare awareness, and growing investments in neuroscience research are expected to sustain market momentum. With increasing adoption of advanced devices across hospitals, diagnostic centers, and outpatient facilities, the industry is well-positioned for long-term growth.
Key Factors Driving the United States Neurology Devices Market Growth
Rising Prevalence of Neurological Disorders
The increasing prevalence of neurological disorders is one of the primary factors driving the U.S. neurology devices market. Conditions such as epilepsy, multiple sclerosis, Alzheimer’s, and Parkinson’s are rising due to aging demographics and lifestyle changes. These disorders require sophisticated diagnostic and therapeutic tools to ensure timely management and improve patient quality of life. Neurology devices such as electroencephalography systems, neuromodulation devices, and advanced imaging technologies are crucial in detecting, monitoring, and treating these conditions effectively. The demand for improved patient outcomes and reduced hospitalization rates is accelerating adoption across healthcare facilities. With the growing burden of neurological diseases on the healthcare system, investments in device innovation and accessibility are intensifying, ensuring sustained demand for advanced neurology devices across the United States.Technological Advancements and Innovation
Technological innovation is significantly driving the growth of the U.S. neurology devices market. Advancements in neurostimulation, neuroimaging, and neurosurgical instruments are enhancing treatment precision and minimizing risks. Minimally invasive neurosurgical techniques supported by robotic systems are becoming more widely available, reducing recovery times and improving surgical outcomes. Wearable neurodiagnostic devices and brain-computer interfaces are opening new possibilities in patient monitoring and rehabilitation. Integration of AI and machine learning with neurology devices is enabling predictive analysis, enhancing early diagnosis, and personalizing treatments. These innovations are not only improving patient care but also reducing the overall healthcare burden. Continuous R&D investments by medical device companies and academic institutions ensure that the industry remains at the forefront of technological breakthroughs, fueling long-term growth.Expanding Healthcare Infrastructure and Investments
Growing healthcare infrastructure and rising investments are major contributors to the U.S. neurology devices market. Hospitals, diagnostic centers, and outpatient facilities are rapidly adopting advanced neurology devices to expand their service capabilities and meet patient demand. Significant funding from government initiatives and private investors is supporting the development and commercialization of novel neurological technologies. The presence of specialized neurology clinics and research institutions across the country further enhances accessibility to cutting-edge solutions. Additionally, increasing awareness about neurological conditions among patients and caregivers is boosting demand for effective devices. Partnerships between healthcare providers and medical device companies are enabling faster adoption and broader reach of innovative solutions. These combined factors are creating a favorable environment for market expansion and sustained innovation across the United States.Challenges in the United States Neurology Devices Market
High Costs and Reimbursement Barriers
The high costs associated with neurology devices remain a significant challenge in the U.S. market. Advanced systems such as neurostimulation implants, robotic neurosurgical devices, and imaging technologies require substantial investment, making them less accessible for smaller healthcare facilities. Reimbursement policies for neurological treatments and procedures often lack consistency, creating uncertainty for both providers and patients. These barriers limit widespread adoption and pose challenges for manufacturers seeking broader market penetration. Smaller and rural hospitals, in particular, face difficulties in integrating costly technologies into their services. Without favorable reimbursement structures and cost-effective device models, patient access to advanced neurology devices remains restricted. Addressing these financial barriers will be essential to expanding adoption and ensuring equitable access to neurological care across the country.Regulatory and Clinical Validation Challenges
Regulatory complexities and the need for extensive clinical validation pose another hurdle for the U.S. neurology devices market. Devices such as neuromodulation systems and implantable neurostimulators must undergo rigorous testing to ensure safety and efficacy, prolonging approval timelines. The lengthy regulatory review process often delays commercialization and increases development costs. Smaller companies face added pressure in navigating these requirements, limiting their ability to compete with larger medical device manufacturers. Additionally, uncertainties around long-term safety outcomes and device durability can hinder acceptance by regulatory authorities and healthcare providers. While necessary for patient safety, these stringent regulations slow innovation and restrict faster access to groundbreaking solutions. Streamlined regulatory frameworks and collaborative clinical trials will be crucial in overcoming these challenges and accelerating market growth.United States Neurology Devices Market Overview by States
Regional growth in the U.S. neurology devices market is led by biotechnology and healthcare hubs, with California, Texas, New York, and Florida driving innovation through research excellence, advanced infrastructure, and expanding healthcare investment networks. The following provides a market overview by States:
California Neurology Devices Market
California represents one of the most prominent neurology devices markets in the United States, driven by its strong healthcare ecosystem and concentration of leading research institutions. The state is home to world-class universities, hospitals, and medical device companies advancing neurological innovation. Silicon Valley’s technology expertise is also contributing to the development of AI-integrated and wearable neurology devices. California’s vibrant startup culture, supported by venture capital and state-level initiatives, is accelerating commercialization of advanced solutions. Hospitals and clinics across the state are early adopters of minimally invasive neurosurgical tools, neurostimulation systems, and diagnostic imaging technologies. With a skilled workforce, robust infrastructure, and strong clinical trial activity, California continues to lead in developing next-generation neurology devices, reinforcing its status as a key contributor to the U.S. market.Texas Neurology Devices Market
Texas is emerging as a rapidly growing hub in the U.S. neurology devices market, supported by its expanding healthcare network and research ecosystem. Major medical centers in Houston, Dallas, and Austin are driving innovation in neurosurgery, neurostimulation, and neurodiagnostic. The state’s lower operational costs compared to other healthcare hubs make it attractive for both startups and established companies seeking to expand manufacturing and R&D operations. Texas-based universities and research hospitals are playing a pivotal role in developing and testing new neurology devices, supported by collaborations with medical device manufacturers. Government-backed healthcare initiatives and private investments are further strengthening market growth. With increasing focus on neurological disease management and clinical adoption of advanced technologies, Texas is positioning itself as a significant contributor to national market expansion.New York Neurology Devices Market
New York holds a strong presence in the U.S. neurology devices market, driven by its concentration of premier hospitals, universities, and research institutions. The state is a hub for clinical trials and translational research, particularly in neuroimaging, neuromodulation, and neurosurgical devices. New York City’s thriving biotechnology ecosystem, supported by significant private and public funding, is accelerating advancements in neurology technologies. Hospitals across the state are integrating advanced diagnostic and therapeutic devices to enhance patient care and outcomes. Collaborative partnerships between research institutions and device manufacturers are fostering innovation in areas such as brain-computer interfaces and minimally invasive surgery. With its advanced healthcare infrastructure and growing demand for neurological solutions, New York remains a key market, contributing significantly to the overall growth of the U.S. neurology devices industry.Florida Neurology Devices Market
Florida is steadily strengthening its role in the U.S. neurology devices market, supported by its expanding healthcare sector and growing focus on neurological care. The state’s hospitals and research institutions are increasingly adopting advanced neurology devices for diagnosis and treatment of conditions such as Alzheimer’s, epilepsy, and stroke. Biotechnology clusters in cities like Miami and Orlando are driving research collaborations and clinical advancements. Florida’s large aging population is fueling demand for innovative devices, particularly in neurostimulation and neurodiagnostic. Supportive state-level policies and investment initiatives are further encouraging medical technology adoption. Additionally, clinical research organizations and healthcare providers are collaborating with device manufacturers to accelerate product development and commercialization. Florida’s strategic focus on healthcare innovation positions it as a competitive market in the U.S. neurology devices industry.Market Segmentations
Product
- Neurostimulation
- Neurosurgery Devices
- Interventional Neurology
- CSF Management Devices
- Others
End Use
- Hospitals
- Ambulatory Surgery Centers
- Others
States
- California
- Texas
- New York
- Florida
- Illinois
- Pennsylvania
- Ohio
- Georgia
- New Jersey
- Washington
- North Carolina
- Massachusetts
- Virginia
- Michigan
- Maryland
- Colorado
- Tennessee
- Indiana
- Arizona
- Minnesota
- Wisconsin
- Missouri
- Connecticut
- South Carolina
- Oregon
- Louisiana
- Alabama
- Kentucky
- Rest of United States
All the Key players have been covered
- Overviews
- Key Person
- Recent Developments
- SWOT Analysis
- Revenue Analysis
Company Analysis:
- Medtronic Plc.
- B. Braun Melsungen AG
- Boston Scientific Corporation
- Stryker Corporation
- Abbott Laboratories
- Johnson and Johnson
- Smith & Nephew
- MicroPort Scientific Corporation
Table of Contents
Companies Mentioned
- Medtronic Plc.
- B. Braun Melsungen AG
- Boston Scientific Corporation
- Stryker Corporation
- Abbott Laboratories
- Johnson and Johnson
- Smith & Nephew
- MicroPort Scientific Corporation
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | August 2025 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 3.57 Billion |
| Forecasted Market Value ( USD | $ 6.32 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | United States |
| No. of Companies Mentioned | 8 |