Key Highlights:
- The North America market dominated Global Dietary Supplement Testing Market in 2024, accounting for a 36% revenue share in 2024.
- The U.S. market is projected to maintain its leadership in North America, reaching a market size of USD 1.03 billion by 2032.
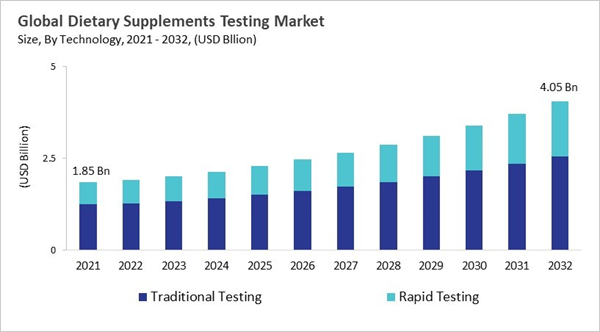
- Among the various Technology, the traditional testing segment dominated the global market, contributing a revenue share of 66% in 2024.
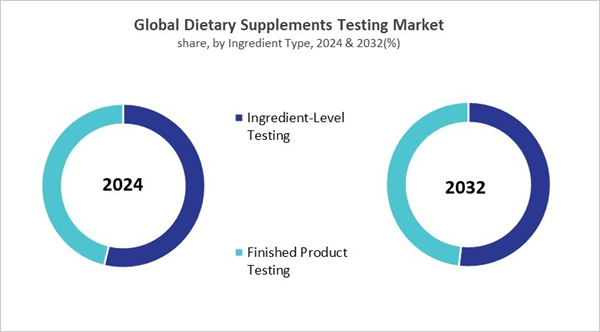
- In terms of Ingredient Type, Ingredient-Level Testing segment are expected to lead the global market, with a projected revenue share of 51.86% by 2032.
- The independent third-party testing laboratories market emerged as the leading Service Provider in 2024, capturing a 65% revenue share, and is projected to retain its dominance during the forecast period.
- The Nutraceutical Companies Market in End User is poised to grow at the market in 2032 with a market size of USD 1.61 billion and is projected to maintain its dominant position throughout the forecast period.
- By Test Type the Contaminants (heavy metals, pesticides, solvents) Segment captured the market size of USD 559.8 million in 2024 and this segment will maintain its position during the forecast period.
The dietary supplement testing market has recently gained traction as a science-driven and mature industry supported by pharmaceutical standards, food safety practices, and rising consumer demand for transparency. In order to ensure that supplements are effective, safe, and accurately labelled, regulatory frameworks like Codex Alimentarius, the FDA’s cGMP in the US. Further, organizations such as the United States Pharmacopeia and AOAC International have defined standardized methods and set scientific benchmarks that ensure regulatory compliance. Other than this, ODM and OEM manufacturers have integrated strong testing systems in production with the aim of meeting international standards. This evolution from manufacturing practices and regulatory science showcases how supplement testing has emerged as an important part of the industry.
The dietary supplement testing market is anticipated to be driven by factors, including regulatory harmonization, technical advancements, and deployment into the production process. Further, portable testing devices are gaining traction as they decentralize quality control, offer real-time results, and supply chain transparency in fast-moving and low-resource environments. Regulatory bodies such as EFSA, FDA and regional governments are developing stringent regulations and supporting international trade while rising compliance needs. Also, ODM suppliers and OEMs are integrating testing within manufacturing, ensuring efficiency and decreasing the compliance risks for global brands. These elements are resulting in a transformation in the market, and key players are competing by the ways of innovations, compliance, collaborations with health organization and NGOs to ensure safety and quality of dietary supplements.
Driving and Restraining Factors
Drivers
- Rising Consumer Awareness Of Health And Wellness
- Stringent Regulatory Standards And Compliance Requirements
- Increasing Incidences Of Adulteration And Safety Concerns
- Technological Advancements In Testing Methods
Restraints
- High Costs Of Comprehensive Testing And Infrastructure Burdens
- Lack Of Standardized And Harmonized Testing Protocols For Emerging Ingredients
- Fragmented And Inadequate Testing Infrastructure In Developing Regions
Opportunities
- Expansion Into Emerging Markets With Growing Supplement Consumption
- Adoption Of Advanced Testing Technologies And Digital Platforms
- Sustainability And Clean Label Movement Driving Testing Demand
Challenges
- Regulatory Complexity And Global Standardization Issues
- Adulteration, Contamination, And Detection Complexity
- Technological, Operational, And Cost Barriers
COVID-19 Impact Analysis
The COVID-19 pandemic significantly heightened consumer awareness regarding health and immunity, driving a surge in demand for dietary supplements. This shift prompted manufacturers to prioritize stringent quality assurance, leveraging third-party laboratories and advanced testing technologies to ensure product safety, authenticity, and efficacy. Concurrently, governments and regulatory bodies reinforced compliance with safety and labeling standards, further propelling the dietary supplement testing market. The industry experienced rapid technological advancements and infrastructure upgrades to support increased production of immunity-boosting supplements, establishing testing services as critical for consumer trust and public health protection. Thus, the COVID-19 pandemic had a positive impact on the market.Technology Outlook
Based on Technology, the market is segmented into Traditional Testing and Rapid Testing. The rapid testing segment attained 34% revenue share in the dietary supplement testing market in 2024. Rapid testing methods allow companies to quickly identify contaminants, verify active ingredient levels, and confirm label claims, all while significantly reducing turnaround times. This is particularly valuable in global supply chains, where manufacturers must respond quickly to evolving consumer demands and regulatory requirements.Ingredient Type Outlook
Based on Ingredient Type, the market is segmented into Ingredient-Level Testing and Finished Product Testing. The finished product testing segment recorded 46% revenue share in the dietary Supplement Testing market in 2024. The finished product testing segment also plays a vital role in maintaining quality across the global dietary supplement industry by validating safety, stability, and effectiveness once supplements are fully manufactured. This includes confirming that products deliver on label claims, retain potency throughout their shelf life, and remain safe under varying storage and transportation conditions.Service Provider Outlook
Based on Service Provider, the market is segmented into Contract Research Organizations (CROs), Independent Third-Party Testing Laboratories, and Other Service Provider. The contract research organizations (CROs) segment witnessed 24% revenue share in the dietary supplement testing market in 2024. CROs are increasingly chosen by companies looking for cost-effective access to high-end testing methods, regulatory consulting, and customized studies such as stability and shelf life testing. They are particularly valuable for small and medium-sized companies that lack the infrastructure for in-house testing but must meet the same rigorous regulatory requirements as larger firms.Regional Outlook
Region-wise, the dietary supplement testing market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment recorded 36% revenue share in the dietary supplement testing market in 2024. The dietary supplement market is estimated to expand at a high rate in the North America and Europe region. This growth is supported by the rising consumer awareness and strict regulations across the regions. Additionally, in Europe, the dietary supplement market’s growth is led by the EFSA’s (European Food Safety Authority) focus towards accurate labelling, risk assessment, and harmonization of safety standards, thereby developing a highly structured testing environment. The increasing focus on preventive healthcare and transparency is resulting in rising investment in better testing technologies and partnerships.The Asia Pacific and LAMEA regions are expected to witness substantial expansion in the dietary supplement testing market. This expansion is supported by the increasing health awareness and rising supplement consumption. The Asia Pacific region has a high presence of supplement manufacturing OEMs and ODMs, particularly in India and China. As a result, in house testing capabilities have become an important element to meet the regulatory demands across the globe. Also, regional governments are positively adopting Codex guidelines to improve consumer safety and international trade. The dietary supplement market is estimated to capture a promising market share driven by the rising investment in preventive healthcare and nutrition programs across the region. Also, NGOs and international bodies are playing an essential role in testing and monitoring fortified foods.
List of Key Companies Profiled
- Eurofins Scientific SE
- Tentamus Group GmbH
- Intertek Group PLC
- Alkemist Labs
- SGS S.A.
- AGROLAB GmbH
- Anresco, Inc.
- FoodChain ID Group, Inc.
- BeaconPointLabs, LLC
- Certified Laboratories, LLC
Market Report Segmentation
By Technology
- Traditional Testing
- Rapid Testing
By Ingredient Type
- Ingredient-Level Testing
- Finished Product Testing
By Service Provider
- Contract Research Organizations (CROs)
- Independent Third-Party Testing Laboratories
- Other Service Provider
By End User
- Nutraceutical Companies
- Contract Manufacturers
- Distributors / Label Claim Verificationers (Online & Offline)
- Regulatory Authorities
- Other End User
By Test Type
- Contaminants (heavy metals, pesticides, solvents)
- Microbiological
- Potency
- Identity / Authentication
- Adulteration
- Label Claim Verification
- Stability & Shelf Life
- Allergen & GMO Testing
- Other Test Types
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Table of Contents
Companies Mentioned
- Eurofins Scientific SE
- Tentamus Group GmbH
- Intertek Group PLC
- Alkemist Labs
- SGS S.A.
- AGROLAB GmbH
- Anresco, Inc.
- FoodChain ID Group, Inc.
- BeaconPointLabs, LLC
- Certified Laboratories, LLC