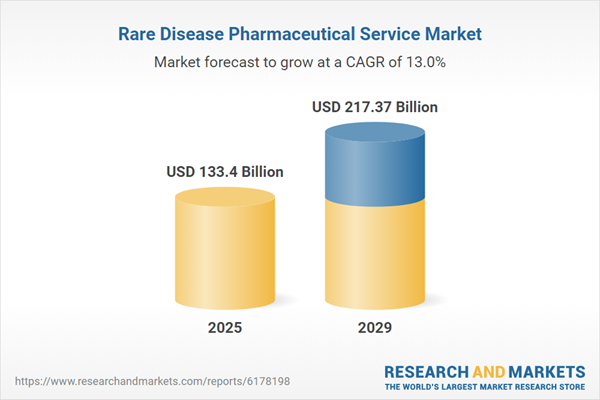
The rare disease pharmaceutical service market size is expected to see rapid growth in the next few years. It will grow to $217.37 billion in 2029 at a compound annual growth rate (CAGR) of 13%. The growth in the forecast period result from the growing application of artificial intelligence in drug discovery, increased demand for personalized treatment approaches, higher investment in rare disease clinical trials, more frequent use of real-world evidence in decision-making, and stronger regulatory support for therapies targeting rare diseases. Key trends in the forecast period include AI-driven drug discovery innovations, adoption of digital health platforms, technology-assisted patient monitoring, development of biomarker-based therapies, and progress in decentralized clinical trials.
The growth of personalized medicine is set to accelerate the expansion of the rare disease pharmaceutical service market. This medical approach customizes treatments based on a patient's genetic profile, lifestyle, and environmental factors, allowing for more precise and effective care. Advances in genomics, biomarker research, and data analytics enable treatments to be tailored specifically to an individual’s genetic makeup, improving treatment outcomes, minimizing side effects, and addressing previously unmet medical needs with greater accuracy than traditional therapies. Pharmaceutical services contribute to this trend by developing targeted therapies, using genetic and biomarker data, and offering tailored solutions for individual patients. For example, in February 2024, the Personalized Medicine Coalition, a US-based non-profit, reported that in 2023, the FDA approved 16 new personalized treatments for rare disease patients, a significant rise from just 6 approvals in 2022. This surge in personalized medicine is driving the growth of the rare disease pharmaceutical service market.
Companies in the rare disease pharmaceutical service market are increasingly focused on developing innovative treatment options, such as acetylleucine-based therapies, to improve the effectiveness of treatments, enhance patient outcomes, and address gaps in the management of rare neurological and metabolic disorders. Acetylleucine, a modified amino acid (N-acetyl-L-leucine), serves as a therapeutic agent to improve motor function, balance, and overall neurological health in affected patients. For example, in September 2024, IntraBio, a US-based biopharmaceutical company, received FDA approval for AQNEURSA, the first standalone treatment for Niemann-Pick Disease Type C, a rare and life-threatening neurodegenerative disorder. This approval is significant, as AQNEURSA showed rapid and meaningful improvements in neurological symptoms, offering a groundbreaking therapeutic option for both pediatric and adult patients with this condition.
In March 2024, AstraZeneca, a UK-based pharmaceutical company, acquired Amolyt Pharma for an undisclosed sum. The goal of this acquisition is to bolster AstraZeneca’s late-stage rare disease pipeline, particularly within its Alexion division, by incorporating novel therapies for rare endocrine disorders. Amolyt Pharma, a clinical-stage biotechnology company based in France, specializes in developing peptide-based treatments for rare endocrine diseases.
Major players in the rare disease pharmaceutical service market are Johnson & Johnson, Merck & Co. Inc., Pfizer Inc., AbbVie Inc., Sanofi S.A., Novartis AG, AstraZeneca PLC, GlaxoSmithKline PLC (GSK), Takeda Pharmaceutical Company Limited, Amgen Inc., Biogen Inc., Chiesi Farmaceutici S.p.A., Recordati Industria Chimica e Farmaceutica S.p.A., BioMarin Pharmaceutical Inc., Sarepta Therapeutics Inc., Ultragenyx Pharmaceutical Inc., Amicus Therapeutics Inc., Travere Therapeutics Inc., Sciensus Healthcare Ltd., and SOM Innovation Biotech S.L.
North America was the largest region in the rare disease pharmaceutical service market in 2024. The regions covered in rare disease pharmaceutical service report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the rare disease pharmaceutical service market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the pharmaceutical companies contend with tariffs on APIs, glass vials, and lab equipment inputs with few alternative sources. Generic drug makers, operating on razor-thin margins, are especially vulnerable, with some reducing production of low-profit medicines. Biotech firms face delays in clinical trials due to tariff-related shortages of specialized reagents. In response, the industry is expanding API production in India and Europe, increasing inventory stockpiles, and pushing for trade exemptions for essential medicines.
Rare disease pharmaceutical services involve specialized efforts to research, develop, and deliver medications for illnesses affecting a very small number of patients. They aim to meet unmet medical needs through new treatments, regulatory guidance, and support for patient care. These services help ensure that patients with rare conditions have access to necessary therapies, can afford them, and maintain consistent treatment.
Key categories of rare disease pharmaceutical services include drug discovery and clinical trials. Drug discovery involves systematically identifying, refining, and testing potential therapeutic compounds. These services are applied in areas such as genetic disorders, cancers, and endocrine conditions. Organizations that utilize these services include pharmaceutical and biotechnology companies, academic and research institutions, hospitals and specialty clinics, contract research and development organizations, as well as diagnostic and genetic testing laboratories.
The rare disease pharmaceutical services market research report is one of a series of new reports that provides rare disease pharmaceutical services market statistics, including rare disease pharmaceutical services industry global market size, regional shares, competitors with a rare disease pharmaceutical services market share, detailed rare disease pharmaceutical services market segments, market trends and opportunities, and any further data you may need to thrive in the rare disease pharmaceutical services industry. This rare disease pharmaceutical services market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The rare disease pharmaceutical service market consists of revenues earned by entities by providing services such as clinical trial management, patient recruitment, regulatory compliance, market access, and safety monitoring. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Rare Disease Pharmaceutical Service Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on rare disease pharmaceutical service market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for rare disease pharmaceutical service? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The rare disease pharmaceutical service market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Types: Drug Discovery; Clinical Trials; Other Types2) By Application: Congenital And Genetic Diseases; Tumors And Cancer; Endocrine Diseases
3) By End User: Pharmaceutical Companies; Biotechnology Companies; Academic And Research Institutions; Hospitals And Specialty Clinics; Contract Research Organizations (CROs) And Contract Development And Manufacturing Organizations (CDMOs); Diagnostic Laboratories And Genetic Testing Centers
Subsegments:
1) By Drug Discovery: Target Identification And Validation; Preclinical Research And Toxicology Studies; Biomarker Discovery And Validation; Genomic And Proteomic Analysis; Computational Drug Design And Modeling; High-Throughput Screening (HTS); Lead Optimization And Candidate Selection2) By Clinical Trials: Clinical Trial Design And Protocol Development; Patient Recruitment And Retention; Site Management And Monitoring; Regulatory Affairs And Compliance Support; Data Management And Biostatistics; Pharmacovigilance And Safety Monitoring; Real-World Evidence (RWE) And Post-Marketing Studies
3) By Other Types: Market Access And Pricing Strategy; Health Economics And Outcomes Research (HEOR); Regulatory Affairs And Compliance Services; Manufacturing And CMC (Chemistry, Manufacturing, and Controls) Support; Medical Affairs And Scientific Communication; Post-Marketing Surveillance; Consulting And Strategic Advisory
Companies Mentioned: Johnson & Johnson; Merck & Co. Inc.; Pfizer Inc.; AbbVie Inc.; Sanofi S.A.; Novartis AG; AstraZeneca PLC; GlaxoSmithKline PLC (GSK); Takeda Pharmaceutical Company Limited; Amgen Inc.; Biogen Inc.; Chiesi Farmaceutici S.p.A.; Recordati Industria Chimica e Farmaceutica S.p.A.; BioMarin Pharmaceutical Inc.; Sarepta Therapeutics Inc.; Ultragenyx Pharmaceutical Inc.; Amicus Therapeutics Inc.; Travere Therapeutics Inc.; Sciensus Healthcare Ltd.; SOM Innovation Biotech S.L.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Rare Disease Pharmaceutical Service market report include:- Johnson & Johnson
- Merck & Co. Inc.
- Pfizer Inc.
- AbbVie Inc.
- Sanofi S.A.
- Novartis AG
- AstraZeneca PLC
- GlaxoSmithKline PLC (GSK)
- Takeda Pharmaceutical Company Limited
- Amgen Inc.
- Biogen Inc.
- Chiesi Farmaceutici S.p.A.
- Recordati Industria Chimica e Farmaceutica S.p.A.
- BioMarin Pharmaceutical Inc.
- Sarepta Therapeutics Inc.
- Ultragenyx Pharmaceutical Inc.
- Amicus Therapeutics Inc.
- Travere Therapeutics Inc.
- Sciensus Healthcare Ltd.
- SOM Innovation Biotech S.L.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 133.4 Billion |
| Forecasted Market Value ( USD | $ 217.37 Billion |
| Compound Annual Growth Rate | 13.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |