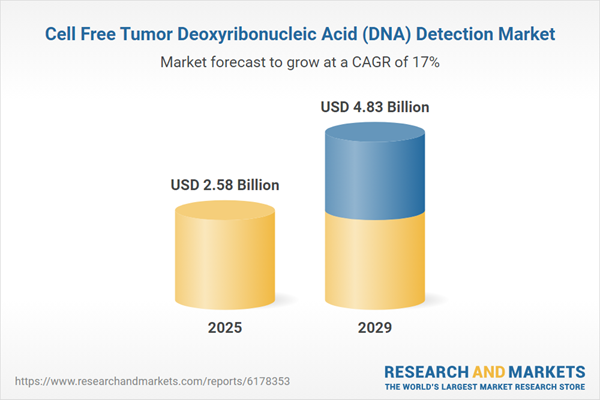
The cell free tumor deoxyribonucleic acid (DNA) detection market size is expected to see rapid growth in the next few years. It will grow to $4.83 billion in 2029 at a compound annual growth rate (CAGR) of 17%. The growth in the forecast period is driven by greater adoption of cell-free tumor DNA-based companion diagnostics, increased use of cell-free tumor DNA testing in clinical trials, rising global awareness of innovative cancer diagnostics, growth in telemedicine and remote patient monitoring, and higher demand for multi-cancer early detection panels. Key trends in the forecast period include advancements in ultra-sensitive cell-free tumor DNA detection methods, development of advanced liquid biopsy platforms for multi-cancer detection, innovation in non-invasive prenatal and oncology diagnostics, creation of point-of-care cell-free tumor DNA testing devices, and progress in minimally invasive disease monitoring techniques.
The growing demand for personalized medicine is expected to contribute to the expansion of the cell free tumor deoxyribonucleic acid detection market in the coming years. Personalized medicine is a healthcare approach that customizes treatment and prevention strategies according to an individual's specific genetic profile, lifestyle, and environmental factors. This approach is gaining momentum due to advancements in genomics, which make it possible to identify genetic variations with precision and create tailored treatment plans. Cell free tumor deoxyribonucleic acid detection supports personalized medicine by offering non-invasive, real-time information about the genetic characteristics of a patient's tumor. It allows healthcare providers to customize treatment based on individual mutation profiles, track therapeutic responses, and identify disease recurrence at an early stage, leading to improved patient outcomes and greater accuracy in cancer care. For example, in February 2024, the Personalized Medicine Coalition, a nonprofit organization based in the United States, reported that the U.S. Food and Drug Administration approved 26 new personalized medicines in 2023, a considerable rise from the 12 approved in 2022. As a result, the increasing demand for personalized medicine is contributing to the growth of the cell free tumor deoxyribonucleic acid detection market.
Leading companies in the cell free tumor deoxyribonucleic acid detection market are focusing on creating advanced products such as library preparation kits that improve the accuracy, sensitivity, and speed of cancer testing. Library preparation kits are reagent products used to prepare DNA or RNA samples for sequencing by breaking them into smaller fragments, tagging them, and amplifying them for more accurate analysis. For instance, in February 2024, Twist Bioscience Corporation, a biotechnology company based in the United States, introduced the cfDNA Library Preparation Kit to improve the sensitivity and accuracy of liquid biopsy applications. This kit allows for efficient generation of cell free DNA libraries suitable for next-generation sequencing on Illumina platforms, helping overcome issues associated with low-input and degraded DNA samples. The goal of this innovation is to enhance the reliability and effectiveness of liquid biopsy testing, supporting earlier cancer detection and better monitoring of treatment responses.
In February 2022, Labcorp Holdings Inc., a healthcare company based in the United States, acquired Personal Genome Diagnostics Inc. for an undisclosed amount. Through this acquisition, Labcorp intends to expand its capabilities in comprehensive genomic profiling and liquid biopsy testing. This move strengthens the company’s position in the precision oncology sector and broadens access to advanced cell free tumor deoxyribonucleic acid detection technologies for cancer diagnosis and treatment monitoring. Personal Genome Diagnostics Inc. is a biotechnology company based in the United States that specializes in providing services related to cell free tumor DNA detection.
Major players in the cell free tumor deoxyribonucleic acid (dna) detection market are F. Hoffmann-La Roche AG, Thermo Fisher Scientific Inc., Labcorp Holdings Inc., Illumina Inc., Sysmex Inostics Inc., Bio-Rad Laboratories Inc., QIAGEN N.V., Natera Inc., Guardant Health Inc., Invitae Corporation, Foundation Medicine Inc, Adaptive Biotechnologies Corporation, Freenome Holdings Inc., Grail Inc., Biodesix Inc, Personal Genome Diagnostics Inc., Agena Bioscience Inc., Menarini Silicon Biosystems SpA, Exosome Diagnostics Inc., and Lucence Health Inc.
North America was the largest region in the cell free tumor deoxyribonucleic acid (DNA) detection market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in cell free tumor deoxyribonucleic acid (DNA) detection report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the cell free tumor deoxyribonucleic acid (DNA) detection market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The fast surge in U.S. tariffs and the trade tensions that followed in spring 2025 are heavily affecting the medical equipment sector, particularly for imported imaging machine components, surgical-grade stainless steel, and plastic disposables. Hospitals and clinics resist price hikes, pressuring manufacturers’ margins. Regulatory hurdles compound the problem, as tariff-related supplier changes often require re-certification of devices, delaying time-to-market. Companies are mitigating risks by dual-sourcing critical parts, expanding domestic production of commoditized items, and accelerating R&D in cost-efficient materials.
Cell-free tumor deoxyribonucleic acid (DNA) detection is a non-invasive diagnostic method that identifies and analyzes small DNA fragments released into the bloodstream by cancer cells. It provides real-time information on genetic mutations, tumor burden, and disease progression without the need for tissue biopsies. The main purpose is to enable early cancer detection, monitor treatment response, and track minimal residual disease or relapse.
The primary technologies used in cell-free tumor deoxyribonucleic acid (DNA) detection are polymerase chain reaction (PCR), next-generation sequencing (NGS), and digital polymerase chain reaction (dPCR). Polymerase chain reaction (PCR) is a technique that rapidly amplifies specific DNA segments, allowing detection of even minute amounts of tumor DNA. Sample sources include plasma, serum, and urine. Applications cover oncology, non-invasive prenatal testing, transplantation, and other areas. Key end-users include hospitals, diagnostic laboratories, academic and research institutions, and related organizations.
The cell free tumor deoxyribonucleic acid (DNA) detection market research report is one of a series of new reports that provides cell free tumor deoxyribonucleic acid (DNA) detection market statistics, including cell free tumor deoxyribonucleic acid (DNA) detection industry global market size, regional shares, competitors with a cell free tumor deoxyribonucleic acid (DNA) detection market share, detailed cell free tumor deoxyribonucleic acid (DNA) detection market segments, market trends and opportunities, and any further data you may need to thrive in the cell free tumor deoxyribonucleic acid (DNA) detection industry. This cell free tumor deoxyribonucleic acid (DNA) detection market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The cell free tumor deoxyribonucleic acid (DNA) detection market consists of revenues earned by entities by providing services such as early cancer screening, liquid biopsy testing, genomic profiling, treatment response monitoring, and personalized therapy selection. The market value includes the value of related goods sold by the service provider or included within the service offering. The cell free tumor deoxyribonucleic acid (DNA) detection market also includes sales of liquid biopsy kits, next-generation sequencing (NGS) platforms, target enrichment kits, bioinformatics and data analysis software, microfluidics-based detection platforms, and automated liquid handling systems. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Cell Free Tumor Deoxyribonucleic Acid (DNA) Detection Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on cell free tumor deoxyribonucleic acid (dna) detection market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for cell free tumor deoxyribonucleic acid (dna) detection? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The cell free tumor deoxyribonucleic acid (dna) detection market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Report Scope
Markets Covered:
1) By Technology: Polymerase Chain Reaction (PCR); Next-Generation Sequencing (NGS); Digital Polymerase Chain Reaction (dPCR)2) By Source: Plasma; Serum; Urine
3) By Application: Oncology; Non-Invasive Prenatal Testing; Transplantation; Other Applications
4) By End-User: Hospitals; Diagnostic Laboratories; Academic And Research Institutes; Other End-Users
Subsegments:
1) By Polymerase Chain Reaction (PCR): Real-Time Polymerase Chain Reaction (qPCR); Multiplex Polymerase Chain Reaction (PCR); Allele-Specific Polymerase Chain Reaction (PCR); High-Resolution Melting (HRM) Analysis; Beaming Polymerase Chain Reaction (PCR)2) By Next-Generation Sequencing (NGS): Whole Genome Sequencing (WGS); Whole Exome Sequencing (WES); Targeted Gene Panels; Ribonucleic Acid Sequencing (RNA-seq); Methylation Sequencing
3) By Digital Polymerase Chain Reaction (dPCR): Droplet Digital Polymerase Chain Reaction (ddPCR); Chip-Based Digital Polymerase Chain Reaction (PCR); Nanofluidic Digital Polymerase Chain Reaction (PCR)
4) By Other Technologies: Microarray-Based Analysis; Mass Spectrometry; Biosensor-Based Detection; Nanopore Sequencing
Companies Mentioned: F. Hoffmann-La Roche AG; Thermo Fisher Scientific Inc.; Labcorp Holdings Inc.; Illumina Inc.; Sysmex Inostics Inc.; Bio-Rad Laboratories Inc.; QIAGEN N.V.; Natera Inc.; Guardant Health Inc.; Invitae Corporation; Foundation Medicine Inc; Adaptive Biotechnologies Corporation; Freenome Holdings Inc.; Grail Inc.; Biodesix Inc; Personal Genome Diagnostics Inc.; Agena Bioscience Inc.; Menarini Silicon Biosystems SpA; Exosome Diagnostics Inc.; Lucence Health Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Cell Free Tumor Deoxyribonucleic Acid (DNA) Detection market report include:- F. Hoffmann-La Roche AG
- Thermo Fisher Scientific Inc.
- Labcorp Holdings Inc.
- Illumina Inc.
- Sysmex Inostics Inc.
- Bio-Rad Laboratories Inc.
- QIAGEN N.V.
- Natera Inc.
- Guardant Health Inc.
- Invitae Corporation
- Foundation Medicine Inc
- Adaptive Biotechnologies Corporation
- Freenome Holdings Inc.
- Grail Inc.
- Biodesix Inc
- Personal Genome Diagnostics Inc.
- Agena Bioscience Inc.
- Menarini Silicon Biosystems SpA
- Exosome Diagnostics Inc.
- Lucence Health Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | October 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.58 Billion |
| Forecasted Market Value ( USD | $ 4.83 Billion |
| Compound Annual Growth Rate | 17.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 20 |