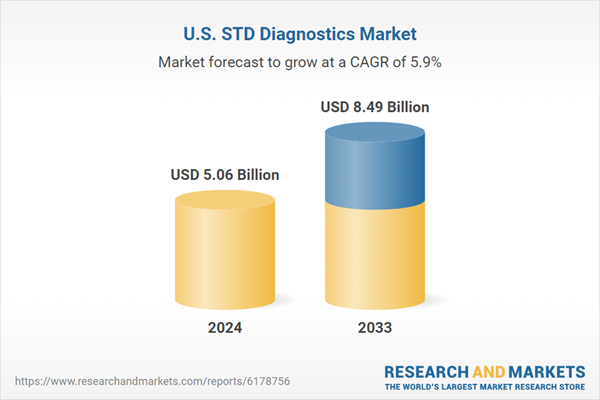
United States STD Diagnostics Industry Overview
Medical tests and techniques used to detect sexually transmitted diseases, including chlamydia, gonorrhea, syphilis, HIV, and HPV, are referred to as STD diagnostics. These diagnostics include quick point-of-care tests that yield results right away as well as laboratory-based testing including molecular assays, immunoassays, and culture procedures. They provide effective treatment, early identification, and transmission prevention. Technological developments have increased the tests' accessibility, speed, and accuracy, allowing for widespread screening and prompt interventions. Controlling the spread of infections, safeguarding the public's health, and lowering problems like infertility, cancer, or systemic disorders associated to untreated infections all depend on STD diagnosis.The market for STD diagnostics in the United States is growing as a result of increased public health awareness, government-led programs encouraging preventive care, and an increase in the prevalence of STDs. Early and accurate detection is improved by increased testing money as well as technical developments including point-of-care tests and molecular diagnostics. Adoption is further accelerated by the growing demand for quick, easily accessible testing, especially among young and high-risk groups. Growth is also fueled by expanded access to healthcare and supportive reimbursement policies. Furthermore, market penetration is strengthened by partnerships between diagnostic firms and healthcare providers as well as by prioritizing routine screenings, which guarantees improved disease management and lower long-term healthcare expenses.
Growth Drivers for the United States STD Diagnostics Market
Rising STD prevalence
One of the main factors driving up demand in the STD diagnostics market is the growing incidence of STDs in the US. More than 2.4 million instances of syphilis, gonorrhea, and chlamydia were reported in 2023, according to the CDC. Even though the overall number of instances decreased by only 2% between 2022 and 2023, the burden is still 13% greater than it was ten years earlier. The rise in congenital syphilis is especially concerning; there were around 4,000 instances in 2024, up for the twelfth consecutive year and roughly 700% more than ten years earlier. All phases of syphilis have seen significant rises in recent years; by 2022, for instance, there will have been an over 80% increase in just five years.Technological Advancements
The market for STD tests in the US is being driven largely by technological advancement. OraSure Technologies' USD 1.5 billion acquisition of Sherlock Biosciences in December 2024 marked a significant turning point in the company's growth in the molecular self-testing market. Through this acquisition, OraSure is able to create a novel self-testing kit for chlamydia and gonorrhea (CT/NG), which is presently undergoing clinical studies and is anticipated to be submitted to the FDA by the end of 2025. The gadget bridges the gap between lab-grade diagnostics and home use by using Sherlock's CRISPR-based and isothermal amplification technologies to provide highly accurate findings from self-collected swabs in less than 30 minutes. These innovations, which decrease diagnostic delays and increase accessibility, are part of a larger industry trend toward quick, point-of-care, and at-home testing solutions. Consumer acceptance of precision molecular diagnostics is anticipated to increase as they become accessible outside of clinical settings. This will support the market's ongoing growth by addressing the demand for early detection and the increased occurrence of infections.Increased Awareness
Another important factor propelling the U.S. STD diagnostics market is rising awareness of sexually transmitted illnesses. Proactive testing is being promoted and stigma is being aggressively reduced by public health campaigns, government programs, and media attention. Programs like the "Get Yourself Tested (GYT)" campaign, which is funded by the CDC, have demonstrated quantifiable success. Research shows that kids who are exposed to the campaign exhibit considerably higher testing intentions and behaviors than those who are not. This tendency is further supported by increased outreach in communities, schools, and colleges, especially among teenagers and young people, who are among the groups most at risk for contracting new illnesses. In addition to raising awareness, social media and digital health platforms have made sexual health education more approachable and relatable. In addition to increasing demand for early and routine testing, this increased awareness also increases adherence to preventive care guidelines. The growth of the U.S. STD diagnostics market is directly fueled by the strengthening diagnostic uptake that occurs as awareness increases.Challenges in the United States STD Diagnostics Market
High Costs
In the US market for STD tests, high prices are a major obstacle. Smaller clinics and low-income populations may find advanced molecular assays, nucleic acid amplification tests (NAATs), and point-of-care fast kits more costly due to their high equipment, reagent, and staffing requirements. Although self-testing and home-based kits are practical, their high cost may prevent widespread use. Many patients are also forced to pay out of pocket due to the huge variations in insurance coverage and reimbursement rates. These financial limitations make it difficult to conduct comprehensive screening, especially in impoverished or rural areas where there may be a high prevalence of STDs but little access to reasonably priced diagnostics. Improving accessibility and promoting early detection across a range of populations requires lowering test costs, increasing insurance coverage, and creating less expensive technology.Regulatory Hurdles
In the US, regulatory barriers provide yet another significant obstacle to STD diagnosis. Every new diagnostic test, particularly molecular and point-of-care devices, must pass a stringent FDA review and approval process, which can be costly and time-consuming. Product development becomes considerably more complex when clinical trial requirements, validation studies, and quality control criteria are included. Regulatory approval delays can delay market access and reduce the availability of cutting-edge diagnostics for pressing public health issues. Administrative difficulties for businesses are further increased by the need to comply with state and federal rules as well as changing standards for lab-developed testing. It can be especially difficult for smaller diagnostic firms to navigate this regulatory environment, which limits their capacity to quickly provide innovative, high-accuracy tests to the marketCalifornia STD Diagnostics Market
California is a key market for STD diagnostics in the United States, driven by its large and diverse population. Urban centers such as Los Angeles and San Francisco face a higher burden of sexually transmitted infections, prompting the need for widespread testing and prevention initiatives. Public health organizations and clinics have implemented advanced diagnostic solutions, including rapid and point-of-care testing, to improve accessibility and early detection. Efforts are focused on underserved and low-income populations, aiming to reduce the risks of untreated infections and associated complications. Additionally, awareness campaigns and community outreach programs play a critical role in encouraging routine screening and reducing stigma. The combination of advanced technology, proactive public health measures, and outreach initiatives positions California as a leading market for STD diagnostics in the country.Texas STD Diagnostics Market
Texas represents a significant market for STD diagnostics, facing unique challenges due to its large population and geographic diversity. Urban areas experience higher infection rates, while rural regions often have limited access to testing facilities. Mobile clinics, community health programs, and partnerships with local organizations are key strategies to increase testing coverage and reach underserved populations. Public awareness campaigns aim to reduce stigma associated with sexually transmitted infections and encourage routine screening. Despite efforts, disparities in healthcare access, infrastructure limitations, and social barriers continue to impact diagnostic adoption. Addressing these challenges through education, technology deployment, and expanded access is critical to improving early detection and treatment outcomes, driving growth in Texas’s STD diagnostic market.New York STD Diagnostics Market
New York, particularly New York City, has a well-established infrastructure for STD diagnostics, with numerous clinics and public health programs aimed at improving accessibility. The state emphasizes routine testing, targeting high-risk populations through educational initiatives and community outreach programs. Free or low-cost diagnostic services are widely available, particularly in urban areas, while rural regions face challenges in service coverage. Ongoing campaigns focus on reducing stigma, promoting sexual health education, and encouraging proactive screening. Partnerships between healthcare providers, local organizations, and public health authorities enhance the reach and effectiveness of these initiatives. The combination of robust infrastructure, educational programs, and targeted outreach strengthens New York’s position as a leading market for STD diagnostics in the United States.Florida STD Diagnostics Market
Florida’s STD diagnostic market is shaped by diverse population centers and varying levels of healthcare access. Public health clinics and community outreach programs are central to increasing testing availability, particularly in underserved areas. Urban regions have concentrated efforts to ensure early detection, while rural communities face challenges in accessing testing services. Public awareness campaigns aim to reduce stigma, educate residents about sexual health, and encourage regular screening. Efforts focus on preventive care, early diagnosis, and timely treatment to minimize the spread of infections. Collaborative initiatives between healthcare providers, state health departments, and community organizations support these goals. Overall, Florida’s approach to expanding access, improving awareness, and implementing targeted diagnostic strategies drives growth in the STD diagnostics market.Recent Developments in United States STD Diagnostics Market
- In January 2025, OraSure Technologies, Inc., announced that the Center for Biologics Evaluation and Research (CBER) of the U.S. Food and Drug Administration (FDA) approved a labeling update for the OraQuick HIV Self-Test. This update broadens the age range of individuals eligible for the test, including adolescents aged 14 and older, whereas it was previously approved for those 17 and older. This change aims to improve access to HIV testing for younger individuals.
- May 2025: Visby Medical submitted its Men’s Sexual Health Test to the FDA for clearance and CLIA waiver, extending its at-home portfolio beyond the Women’s kit approved two months earlier.
- In February 2023, Thermo Fisher Scientific announced the launch of its Applied Biosystems TrueMark STI select panel, a polymerase chain reaction (PCR) research used to detect neisseria gonorrhoeae, mycoplasma genitalium, chlamydia trachomatis, and trichomonas vaginalis in a single test. This launch expanded company’s portfolio of diagnostic solutions and provided a competitive edge within the market.
United States STD Diagnostics Market Segments:
Test Type
- Chlamydia Testing
- Gonorrhea Testing
- Syphilis Testing
- HPV Testing
- HSV Testing
- HIV Testing
- Trichomonas Testing
- Mycoplasma genitalium Testing
- Chancroid Testing
Technology
- Immunoassay‐based Methods
- Molecular Diagnostics
- Next-Generation Sequencing
- Biosensor / Microfluidics & Other Emerging Platforms
Location of Testing
- Central & Hospital Laboratories
- Rapid Point-of-Care Platforms
- Over-the-Counter / Home Self-Testing
End User
- Hospitals & Clinics
- Diagnostic Laboratories
- Home Care / OTC
States - Market breakup in 29 viewpoints:
- California
- Texas
- New York
- Florida
- Illinois
- Pennsylvania
- Ohio
- Georgia
- New Jersey
- Washington
- North Carolina
- Massachusetts
- Virginia
- Michigan
- Maryland
- Colorado
- Tennessee
- Indiana
- Arizona
- Minnesota
- Wisconsin
- Missouri
- Connecticut
- South Carolina
- Oregon
- Louisiana
- Alabama
- Kentucky
- Rest of United States
All companies have been covered from 5 viewpoints:
- Company Overview
- Key Persons
- Recent Development & Strategies
- SWOT Analysis
- Sales Analysis
Key Players Analysis
- Abbott Laboratories
- F. Hoffmann-La Roche AG
- Hologic Inc.
- Becton Dickinson and Company
- Danaher Corporation (Cepheid)
- Siemens Healthineers AG
- bioMérieux SA
- Thermo Fisher Scientific Inc.
- Qiagen N.V.
- Bio-Rad Laboratories Inc.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- F. Hoffmann-La Roche AG
- Hologic Inc.
- Becton Dickinson and Company
- Danaher Corporation (Cepheid)
- Siemens Healthineers AG
- bioMérieux SA
- Thermo Fisher Scientific Inc.
- Qiagen N.V.
- Bio-Rad Laboratories Inc.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2025 |
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 5.06 Billion |
| Forecasted Market Value ( USD | $ 8.49 Billion |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |