United States Radioimmunoassay Industry Overview
Radiolabeled molecules are used in Radioimmunoassays (RIAs), which are very sensitive and specific tests for measuring the concentrations of antigen or antibody in a sample. In order to precisely measure extremely small amounts of material, occasionally in the picogram range, the laboratory technique known as radioimmunoassay depends on the specificity of antigen-antibody interactions and the sensitive detection of radioisotopes. Because RIAs are sensitive and can identify hormones in biological fluids at nanomolar and picomolar concentrations, they are used in a comparatively wide range of applications. RIA is now one of the primary methods of evaluation in a clinical laboratory for diagnostic purposes, such as determining the plasma concentrations of the majority of hormones, checking donated blood for the hepatitis B surface antigen (HBsAg), screening for certain drugs of abuse, and related tasks. Applications for RIAs include the treatment of peptic ulcer illness, the early detection of cancer, neurotransmitter research, the identification of infectious agent genera, the detection of allergens in dust and food, and many more.The increased awareness of chronic diseases like cancer and thyroid disorders, which radioimmunoassay is frequently used to monitor, has helped the U.S. dominate the RIA market. Establishing these diagnostic techniques and continuing to develop in healthcare delivery is made relatively simple by a workforce that is technologically savvy and well-educated, as well as by the reliance on academic institutions for joint research. Because of innovation prompted by value provided and clinical-level requirements, the United States is still leading this business.
For instance, the U.S. Department of Energy's Isotope Program announced in February 2024 that the Oak Ridge National Laboratory (ORNL) was the first domestic supplier to start producing Iridium-192 (Ir-192), a significant and commonly used radioisotope in the industrial and medical fields. With the ultimate goal of reducing the U.S. dependency on foreign suppliers, regardless of the industry or sector, this will be the first Ir-192 produced domestically in almost 20 years. Diagnostic enterprises in the RIA sector can scale sensitive assay methods to assure consistent manufacturing of test kits and product R&D since they can reduce their dependency on foreign suppliers for isotopes.
Key Factors Driving the United States Radioimmunoassay Market Growth
Rising Prevalence of Chronic and Hormonal Disorders
The growing burden of chronic and hormonal disorders in the United States is a key driver of the radioimmunoassay market. Conditions such as thyroid dysfunction, diabetes, infertility, and endocrine abnormalities require accurate hormone level assessments, for which RIA offers high sensitivity and specificity. Healthcare providers rely on RIA for precise quantification of hormones and biomarkers that guide treatment planning. The aging population and lifestyle-related diseases have amplified the need for early detection tools. As awareness of preventive healthcare increases, demand for reliable diagnostic testing continues to grow. RIA’s ability to detect low analyte concentrations makes it indispensable in clinical endocrinology and metabolic research. With the expanding diagnostic network and focus on precision medicine, the use of radioimmunoassay for chronic disease management remains a major growth driver in the U.S. market.Advancements in Laboratory Automation and Assay Design
Technological innovations in laboratory automation and assay development are significantly enhancing the performance of radioimmunoassay techniques. Automated platforms have reduced manual errors, improved throughput, and shortened turnaround times, making RIA more efficient for large-scale clinical testing. Modern assay kits now feature enhanced binding efficiency, reduced radioactive exposure, and greater reproducibility. Innovations in radiolabeling, data management, and safety protocols have increased laboratory adoption, particularly in research and hospital settings. Furthermore, improved calibration standards and digital data integration support consistent test accuracy. These advancements allow laboratories to handle diverse analyte panels across clinical chemistry, endocrinology, and pharmaceutical research. As laboratories focus on automation and quality assurance, RIA continues to play an integral role in delivering accurate diagnostic insights, thereby contributing to the U.S. market’s technological evolution.Expanding Research Applications and Pharmaceutical Development
Radioimmunoassay plays a critical role in clinical and pharmaceutical research due to its precision in detecting trace molecules. In drug discovery and pharmacokinetic studies, RIA helps evaluate compound efficacy, metabolism, and safety profiles. Research institutions and biopharmaceutical companies rely on RIA for hormone assays, receptor binding studies, and biomarker validation. The increasing collaboration between academia and industry has accelerated the adoption of RIA across diverse applications. Moreover, funding initiatives supporting biomedical research are boosting demand for validated analytical techniques. RIA’s long-standing credibility and accuracy make it a preferred method for standardizing laboratory results and ensuring data reliability. As the U.S. research ecosystem continues to expand, the use of radioimmunoassay in clinical trials, diagnostic test development, and regulatory submissions is expected to rise, reinforcing its market importance.Challenges in the United States Radioimmunoassay Market
Stringent Regulatory Framework and Safety Concerns
The handling, storage, and disposal of radioactive materials used in radioimmunoassay pose significant regulatory and safety challenges. Laboratories are required to comply with strict federal and state-level guidelines to ensure radiation safety and environmental protection. These compliance requirements often increase operational complexity and costs. Facilities must invest in trained personnel, secure infrastructure, and proper waste management systems, creating barriers for smaller laboratories. Additionally, increasing regulatory scrutiny on radioactive substances limits flexibility in assay development and usage. Concerns over radiation exposure and safety protocols also discourage adoption in certain clinical settings. Although improved radiolabeling techniques and automation have mitigated some risks, compliance burdens remain a major restraint. Simplifying regulations and advancing non-hazardous assay alternatives could help address these concerns and support broader RIA adoption in the U.S. market.Competition from Non-Radioactive Immunoassay Alternatives
The growing adoption of non-radioactive immunoassay methods such as ELISA and CLIA poses a significant challenge to the radioimmunoassay market. These alternatives offer comparable accuracy, reduced safety risks, and easier regulatory compliance, making them more appealing to modern laboratories. Additionally, ELISA and CLIA require simpler infrastructure and less hazardous waste management, lowering operational costs. As technological advancements enhance the sensitivity of non-radioactive assays, many laboratories are transitioning toward these options. This shift is further supported by manufacturers focusing on automation-friendly and high-throughput formats. Despite RIA’s superior precision in certain applications, the convenience, safety, and efficiency of newer immunoassay technologies continue to pressure its market share. Sustaining RIA’s relevance will require continuous innovation, improved assay safety, and cost-effective solutions tailored to specialized diagnostic and research needs.United States Radioimmunoassay Market Overview by States
California, Texas, New York, and Florida lead the U.S. market, supported by advanced healthcare infrastructure, research institutions, and biotechnology presence, driving demand for precise and reliable diagnostic testing technologies across clinical and research applications. The following provides a market overview by States:California Radioimmunoassay Market
California holds a dominant position in the United States radioimmunoassay market due to its extensive network of research institutions, universities, and biotechnology companies. The presence of advanced diagnostic laboratories and funding for biomedical research supports RIA adoption. Growing awareness of endocrine and metabolic disorders has increased clinical testing demand, while the state’s innovation ecosystem fosters collaboration between academia and industry. Integration of automated laboratory systems and adherence to safety protocols have improved efficiency and compliance. Furthermore, investments in healthcare technology and life sciences research enhance the state’s capacity for precise diagnostic testing. Although regulatory complexities and competition from modern immunoassays pose challenges, California’s strong research infrastructure and skilled workforce continue to drive steady RIA utilization across clinical, academic, and industrial laboratories.Texas Radioimmunoassay Market
Texas represents a rapidly expanding market for radioimmunoassay services, supported by its robust healthcare infrastructure and growing medical research activities. Major metropolitan areas such as Houston and Dallas are home to prominent hospitals and life sciences institutions conducting hormone and drug-level studies. The adoption of RIA is driven by increasing prevalence of chronic and hormonal disorders and the need for sensitive diagnostic tools. The state’s favorable research environment and availability of skilled professionals further enhance laboratory capabilities. However, compliance with radioactive safety regulations and cost-intensive equipment maintenance remain challenges. Collaborations between medical centers and academic research facilities continue to promote technological innovation and application diversification. As investment in healthcare and diagnostics grows, Texas is positioned as a key contributor to the overall development of the U.S. radioimmunoassay market.New York Radioimmunoassay Market
New York’s strong presence in medical research and diagnostics makes it a vital hub for the radioimmunoassay market in the United States. The state hosts leading healthcare institutions and research laboratories engaged in endocrinology and pharmaceutical testing. Rising awareness of precision diagnostics and early disease detection has fueled adoption across hospitals and specialty clinics. Academic collaborations and government-funded research initiatives further contribute to market growth. The high concentration of biotechnology firms and availability of skilled laboratory professionals support continuous technological advancement. While operational costs and stringent radioactive handling regulations present barriers, growing investment in diagnostic innovation offsets these challenges. The integration of automated RIA systems and enhanced safety standards ensures continued adoption across clinical and research environments in New York.Florida Radioimmunoassay Market
Florida’s radioimmunoassay market is expanding due to its growing healthcare network, aging population, and increasing demand for advanced diagnostic testing. The prevalence of chronic conditions, including thyroid and metabolic disorders, is driving adoption of sensitive analytical methods like RIA. The state’s focus on clinical research and academic partnerships supports continuous development and utilization of assay-based testing. Additionally, expanding laboratory infrastructure and availability of trained personnel strengthen market potential. Despite regulatory challenges related to radioactive material management, ongoing improvements in safety practices and automation are facilitating broader acceptance. Hospitals, diagnostic centers, and research institutions are investing in precision testing tools to enhance patient outcomes. As healthcare modernization progresses, Florida is emerging as a key regional market contributing to the overall growth of the U.S. radioimmunoassay industry.Recent Developments in U.S. Radioimmunoassay Market
- The automated chemiluminescence immunoassay (ChLIA) test developed by Euroimmun for the direct quantitative determination of free testosterone levels in serum or plasma received 510(k) clearance from the U.S. Food and Drug Administration in January 2025. This is the first assay of its kind to receive FDA approval. It uses iSYS or i10 platforms to provide fast results in 48 minutes, greatly increasing the speed and accuracy of diagnosis for hormonal disorders like PCOS and hypogonadism.
Market Segmentations
Product
- Analyzers
- Reagents and Kits
Application
- Research
- Clinical Diagnostics
End User
- Hospitals
- Clinical Diagnostic Laboratories
- Pharmaceutical Industries
- Other
States
- California
- Texas
- New York
- Florida
- Illinois
- Pennsylvania
- Ohio
- Georgia
- New Jersey
- Washington
- North Carolina
- Massachusetts
- Virginia
- Michigan
- Maryland
- Colorado
- Tennessee
- Indiana
- Arizona
- Minnesota
- Wisconsin
- Missouri
- Connecticut
- South Carolina
- Oregon
- Louisiana
- Alabama
- Kentucky
- Rest of United States
All the Key players have been covered
- Overviews
- Key Persons
- Recent Developments
- SWOT Analysis
- Revenue Analysis
Company Analysis:
- Danaher (Beckman Coulter, Inc.)
- Berthold Technologies GmbH & Co. KG
- DIAsource ImmunoAssays SA
- BioCheck, Inc. (DRG International Inc.)
- IBL International
- Merck KGaA
- Abbexa
- Institute of Isotopes Co. Ltd.
- Marin Biologic Laboratories, Inc.
- Demeditec Diagnostics GmbH
Table of Contents
Companies Mentioned
- Danaher (Beckman Coulter, Inc.)
- Berthold Technologies GmbH & Co. KG
- DIAsource ImmunoAssays SA
- BioCheck, Inc. (DRG International Inc.)
- IBL International
- Merck KGaA
- Abbexa
- Institute of Isotopes Co. Ltd.
- Marin Biologic Laboratories, Inc.
- Demeditec Diagnostics GmbH
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2025 |
| Forecast Period | 2024 - 2033 |
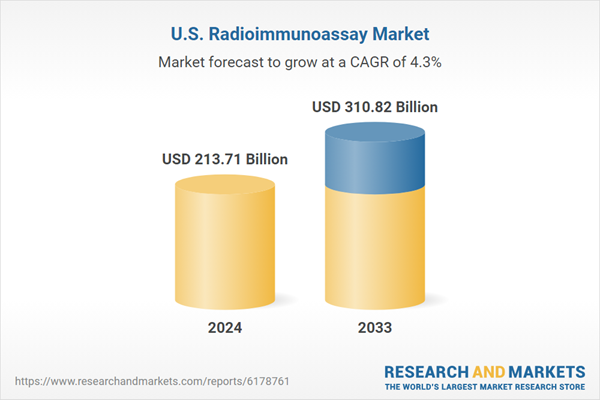
| Estimated Market Value ( USD | $ 213.71 Billion |
| Forecasted Market Value ( USD | $ 310.82 Billion |
| Compound Annual Growth Rate | 4.2% |
| Regions Covered | United States |
| No. of Companies Mentioned | 10 |