Induced Pluripotent Stem Cells Industry Overview
The Induced Pluripotent Stem Cells (iPSC) market is experiencing significant growth due to increasing research in regenerative medicine, personalized therapies, and drug discovery. iPSCs, reprogrammed from adult somatic cells, possess the ability to differentiate into various cell types, making them valuable for studying disease mechanisms and developing customized treatment solutions. The absence of ethical concerns associated with embryonic stem cells has enhanced iPSC adoption across academic and industrial research. Rising demand for cell-based therapies, coupled with advancements in gene editing and reprogramming technologies, is fueling market expansion. Pharmaceutical and biotech companies are increasingly using iPSC-derived models for toxicity testing and drug screening, offering accurate human-based alternatives to animal testing. Continuous technological improvements and enhanced reprogramming efficiency have strengthened the scalability and reliability of iPSC production for diverse applications.The market growth is also driven by expanding applications in precision medicine, disease modeling, and tissue engineering. Public and private sector investments in stem cell research have accelerated scientific innovation and clinical translation. Collaborations among universities, biotech firms, and healthcare institutions are enabling faster development of iPSC-based therapeutic solutions. The growing integration of iPSCs with artificial intelligence and automation technologies is improving manufacturing efficiency and cell characterization accuracy. Furthermore, advancements in culture systems, cryopreservation, and differentiation protocols have improved cell quality and reproducibility, supporting large-scale research initiatives. The availability of government funding and favorable policies in key regions continues to foster innovation in iPSC development, positioning it as a transformative tool in biomedical sciences.
Despite promising prospects, the iPSC market faces challenges such as high production costs, complex manufacturing processes, and stringent regulatory requirements. Standardization in reprogramming methods and cell quality assessment remains a major concern for researchers and developers. Variability in differentiation outcomes and potential genetic instability during reprogramming can limit clinical applications. Additionally, lengthy approval timelines and ethical considerations related to gene manipulation present barriers for commercialization. However, increasing focus on automation, cost reduction, and harmonized regulatory frameworks is expected to overcome these hurdles. With the rising demand for personalized treatment and regenerative solutions, the iPSC market is poised for sustained growth, offering vast potential for innovation in drug discovery, tissue regeneration, and disease research worldwide.
Key Factors Driving the Induced Pluripotent Stem Cells Market Growth
Growing Demand for Regenerative Medicine
The demand for regenerative medicine is a key driver of the Induced Pluripotent Stem Cells market. iPSCs have the ability to differentiate into multiple cell types, allowing them to replace damaged tissues and treat chronic diseases. Their versatility supports the development of cell-based therapies for neurological, cardiac, and metabolic disorders. Unlike embryonic stem cells, iPSCs eliminate ethical issues while offering similar therapeutic potential. Increasing clinical trials and R&D investments in tissue engineering and organ regeneration are strengthening their application scope. Hospitals and research institutions are leveraging iPSC-based technologies for personalized therapies, enhancing treatment accuracy and patient outcomes. Continuous improvements in reprogramming efficiency, coupled with collaborations between academia and biotech firms, are facilitating wider clinical adoption. As healthcare systems shift toward precision medicine, iPSCs are expected to remain central to regenerative and therapeutic innovation.Technological Advancements in Reprogramming and Differentiation
Advancements in reprogramming methods and differentiation protocols are significantly boosting the Induced Pluripotent Stem Cells market. Innovative non-integrating reprogramming techniques have minimized genomic instability risks, improving the safety and consistency of iPSC generation. The integration of automation and bioprocess optimization has enhanced scalability, enabling large-scale production for research and clinical use. Additionally, improved culture media formulations and three-dimensional cell culture technologies have increased efficiency in maintaining pluripotency and directing cell fate. Artificial intelligence and bioinformatics tools are also being used to refine differentiation pathways and ensure reproducibility across batches. Furthermore, advancements in gene editing, such as CRISPR-Cas9, have expanded iPSC applications in disease modeling and drug discovery. As laboratories continue to optimize production and reduce costs, these technological innovations are expected to drive broader adoption across academic, pharmaceutical, and clinical settings.Expanding Applications in Drug Discovery and Disease Modeling
Expanding use of iPSCs in drug discovery and disease modeling is a major driver of market growth. iPSC-derived human cells enable precise modeling of genetic disorders, allowing researchers to test drug efficacy and toxicity under physiologically relevant conditions. This approach enhances predictive accuracy compared to traditional models, reducing development time and costs. Pharmaceutical companies are increasingly integrating iPSC-based assays for high-throughput screening and personalized medicine research. Disease-specific iPSC lines facilitate the study of complex pathologies and rare diseases, improving treatment development. Additionally, regulatory agencies’ growing acceptance of stem cell-based testing platforms supports wider industry adoption. Partnerships between biotech firms and academic institutions are fostering innovation in developing disease-relevant models for cardiovascular, neurological, and metabolic disorders. As precision medicine continues to evolve, iPSCs will remain integral to next-generation drug research and development strategies.Challenges in the Induced Pluripotent Stem Cells Market
High Cost and Production Complexity
The high cost and complexity of iPSC production present a significant challenge for market growth. Generating iPSCs requires advanced technologies, specialized equipment, and skilled expertise, driving up research and manufacturing expenses. Ensuring consistent cell quality, stability, and differentiation accuracy demands extensive testing and quality control, further increasing costs. Large-scale production remains technically challenging due to variability in reprogramming efficiency and culture conditions. Small research institutions and emerging biotech firms often face financial constraints, limiting accessibility to iPSC technologies. Additionally, the need for sterile, regulated environments and long development timelines contributes to operational inefficiencies. While ongoing advancements in automation and cost optimization are helping reduce expenses, achieving affordable large-scale manufacturing remains a priority. Addressing these production challenges is essential to accelerating commercialization and supporting broader adoption in therapeutic and research applications.Regulatory and Standardization Barriers
Regulatory complexities and lack of global standardization pose major challenges to the iPSC market. Variations in international regulatory frameworks make it difficult for developers to achieve approvals for clinical applications. iPSC-based products require extensive validation to ensure genetic stability, differentiation accuracy, and long-term safety, prolonging approval timelines. The absence of standardized quality benchmarks and testing protocols across countries leads to inconsistencies in product development. Moreover, ethical debates surrounding genetic manipulation and patient consent further complicate compliance efforts. Intellectual property issues related to reprogramming techniques also create barriers for market entry and collaboration. While regulatory agencies are gradually introducing guidelines to govern stem cell therapies, regional disparities persist. Establishing harmonized international standards, transparent ethical frameworks, and clear approval pathways will be crucial to promoting innovation and enabling safer, faster commercialization of iPSC-based products.Induced Pluripotent Stem Cells Market Overview by Regions
The iPSC market shows strong growth globally, led by North America’s advanced research infrastructure, Europe’s supportive policies, Asia-Pacific’s expanding biotechnology investments, and emerging interest in regenerative medicine across the Middle East and other developing regions. The following provides a market overview by region:United States Induced Pluripotent Stem Cells Market
The United States dominates the global iPSC market, supported by strong research infrastructure, robust funding, and a thriving biotechnology ecosystem. Leading universities and pharmaceutical firms are driving advancements in iPSC-based drug discovery, regenerative medicine, and disease modeling. Government-backed programs and public-private partnerships encourage innovation, fostering translational research and commercialization. The growing demand for personalized healthcare solutions and precision therapies accelerates clinical adoption across neurological, cardiac, and autoimmune disorders. Additionally, the presence of major biotech companies and advanced manufacturing capabilities strengthens market competitiveness. However, challenges such as high R&D costs and supply chain constraints remain. Continuous technological improvements, combined with supportive regulatory initiatives, are enabling scalable production and broader clinical applications. As innovation in reprogramming, automation, and gene editing advances, the U.S. is expected to maintain its leadership in the global iPSC landscape.United Kingdom Induced Pluripotent Stem Cells Market
The United Kingdom represents a key hub for iPSC research, supported by government funding, academic excellence, and strategic collaborations across the life sciences sector. National initiatives focused on regenerative medicine and precision healthcare drive continuous innovation in iPSC development. Research institutions and biotech firms are leveraging iPSC-derived models for drug screening, toxicity testing, and disease modeling. The UK’s strong regulatory framework ensures ethical and safe research practices, promoting international partnerships. Despite uncertainties around cross-border collaborations and funding post-Brexit, the country remains committed to advancing cellular reprogramming technologies. Integration of automation and AI tools in stem cell research is further enhancing scalability and efficiency. With a robust translational research ecosystem and growing industrial partnerships, the UK continues to play a leading role in Europe’s iPSC advancements and therapeutic applications.China Induced Pluripotent Stem Cells Market
China’s iPSC market is expanding rapidly, driven by government initiatives, large-scale R&D investments, and a growing focus on precision medicine. The establishment of dedicated stem cell research centers and biomanufacturing facilities has strengthened the country’s innovation ecosystem. iPSCs are increasingly used in drug testing, regenerative therapies, and disease modeling, aligning with national healthcare modernization goals. Policy reforms supporting clinical research and technology transfer have attracted domestic and international collaborations. However, challenges related to IP protection and quality standardization persist. The country’s rapidly aging population and rising incidence of chronic diseases are creating new demand for iPSC-based therapeutic solutions. Continued advancements in reprogramming, bioprocessing, and automation technologies are expected to enhance production efficiency. With strong government backing and expanding biotech capabilities, China is positioned as a major growth contributor to the global iPSC industry.United Arab Emirates Induced Pluripotent Stem Cells Market
The United Arab Emirates is emerging as a promising market for iPSC research, supported by strategic government initiatives, healthcare innovation programs, and investments in regenerative medicine. The nation’s growing biotechnology ecosystem encourages collaborations between hospitals, universities, and global research institutions. Focused efforts to establish advanced stem cell centers in Dubai and Abu Dhabi are driving adoption of iPSC-based technologies for therapeutic and diagnostic applications. Partnerships with international biotech firms facilitate technology transfer and skill development. However, limited local manufacturing capabilities and a small research workforce present challenge. Ongoing national strategies aimed at promoting precision medicine and sustainable healthcare are expected to accelerate adoption. As the UAE strengthens its position as a regional innovation hub, the integration of iPSCs into clinical research and personalized medicine is set to advance steadily in the coming years.Recent Developments in Induced Pluripotent Stem Cells Industry
- To increase the effectiveness of iPSC storage and transportation, Pluristyx and Teknova introduced the PluriFreeze cryopreservation technology in March 2025.
- At the BIO International Convention in June 2024, FUJIFILM showcased its enhanced iPSC technology options, emphasizing their suitability for scale-up.
Market Segmentations
Derived Cell Type
- Cardiomyocytes
- Neurons
- Hepatocytes
- Fibroblasts
- Keratinocytes
- Other Cell Types
Application
- Drug Discovery and Development
- Disease Modeling
- Toxicity Testing
- Regenerative Medicine
- Cell Therapy
- Tissue Engineering
- Other
End User
- Academic and Research Institutes
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations
- Hospitals and Specialty Clinics
- Others
Regional Outlook
North America
- United States
- Canada
Europe
- France
- Germany
- Italy
- Spain
- United Kingdom
- Belgium
- Netherlands
- Turkey
Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Malaysia
- Indonesia
- Australia
- New Zealand
Latin America
- Brazil
- Mexico
- Argentina
Middle East & Africa
- Saudi Arabia
- United Arab Emirates
- South Africa
All the Key players have been covered
- Overviews
- Key Persons
- Recent Developments
- SWOT Analysis
- Revenue Analysis
Company Analysis:
- FUJIFILM Cellular Dynamics, Inc.
- Thermo Fisher Scientific, Inc.
- Evotec SE
- ViaCyte Inc.
- Sumitomo Pharma Co. Ltd.
- Takara Bio Inc.
- Fate Therapeutics Inc.
- Ncardia BV
- Axol Bioscience Ltd.
- Cynata Therapeutics Ltd.
Table of Contents
Companies Mentioned
- FUJIFILM Cellular Dynamics, Inc.
- Thermo Fisher Scientific, Inc.
- Evotec SE
- ViaCyte Inc.
- Sumitomo Pharma Co. Ltd.
- Takara Bio Inc.
- Fate Therapeutics Inc.
- Ncardia BV
- Axol Bioscience Ltd.
- Cynata Therapeutics Ltd.
Methodology
In this report, for analyzing the future trends for the studied market during the forecast period, the publisher has incorporated rigorous statistical and econometric methods, further scrutinized by secondary, primary sources and by in-house experts, supported through their extensive data intelligence repository. The market is studied holistically from both demand and supply-side perspectives. This is carried out to analyze both end-user and producer behavior patterns, in the review period, which affects price, demand and consumption trends. As the study demands to analyze the long-term nature of the market, the identification of factors influencing the market is based on the fundamentality of the study market.
Through secondary and primary researches, which largely include interviews with industry participants, reliable statistics, and regional intelligence, are identified and are transformed to quantitative data through data extraction, and further applied for inferential purposes. The publisher's in-house industry experts play an instrumental role in designing analytic tools and models, tailored to the requirements of a particular industry segment. These analytical tools and models sanitize the data & statistics and enhance the accuracy of their recommendations and advice.
Primary Research
The primary purpose of this phase is to extract qualitative information regarding the market from the key industry leaders. The primary research efforts include reaching out to participants through mail, tele-conversations, referrals, professional networks, and face-to-face interactions. The publisher also established professional corporate relations with various companies that allow us greater flexibility for reaching out to industry participants and commentators for interviews and discussions, fulfilling the following functions:
- Validates and improves the data quality and strengthens research proceeds
- Further develop the analyst team’s market understanding and expertise
- Supplies authentic information about market size, share, growth, and forecast
The researcher's primary research interview and discussion panels are typically composed of the most experienced industry members. These participants include, however, are not limited to:
- Chief executives and VPs of leading corporations specific to the industry
- Product and sales managers or country heads; channel partners and top level distributors; banking, investment, and valuation experts
- Key opinion leaders (KOLs)
Secondary Research
The publisher refers to a broad array of industry sources for their secondary research, which typically includes, however, is not limited to:
- Company SEC filings, annual reports, company websites, broker & financial reports, and investor presentations for competitive scenario and shape of the industry
- Patent and regulatory databases for understanding of technical & legal developments
- Scientific and technical writings for product information and related preemptions
- Regional government and statistical databases for macro analysis
- Authentic new articles, webcasts, and other related releases for market evaluation
- Internal and external proprietary databases, key market indicators, and relevant press releases for market estimates and forecasts

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | September 2025 |
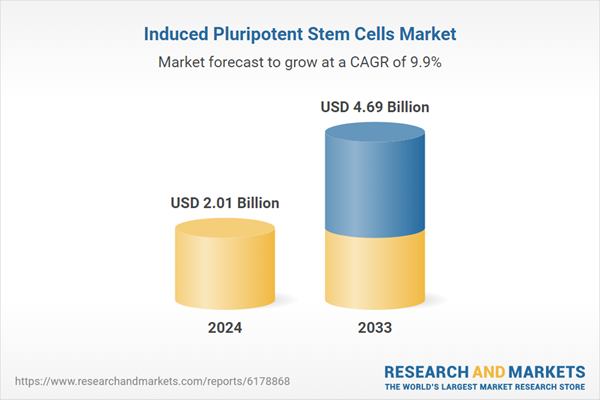
| Forecast Period | 2024 - 2033 |
| Estimated Market Value ( USD | $ 2.01 Billion |
| Forecasted Market Value ( USD | $ 4.69 Billion |
| Compound Annual Growth Rate | 9.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |