Speak directly to the analyst to clarify any post sales queries you may have.
Setting the Stage for Tomorrow's Healthcare: Unveiling the Strategic Imperatives and Market Dynamics Driving the Disposable Medical Device Industry
Rapid advancements in healthcare have elevated the role of disposable medical devices as indispensable assets for modern clinical practice. Over the past decade, the sector has witnessed a convergence of technological breakthroughs and heightened regulatory scrutiny aimed at improving patient safety. This introduction provides an overview of the fundamental forces shaping the industry's trajectory, from evolving clinical protocols to shifting patient expectations.Moreover, infection control imperatives have driven widespread adoption of single-use solutions across hospital settings and outpatient facilities alike. As providers seek to minimize cross-contamination risks, demand for sterile consumables has surged. At the same time, manufacturers are investing heavily in research and development to integrate antimicrobial coatings and ergonomic designs that enhance both clinical efficacy and user comfort.
Looking ahead, the disposable medical device landscape will continue to be influenced by a complex interplay of cost containment pressures and innovation mandates. Stakeholders must balance the imperative for affordability with the need to comply with stringent safety standards. This introduction lays the groundwork for a comprehensive analysis of the market's transformative trends, regulatory shifts, and strategic imperatives that will define the next chapter in patient care delivery.
Navigating Breakthrough Innovations and Evolving Policies that Are Redefining Competitive Landscapes and Patient Care in the Disposable Medical Device Arena
Recent years have ushered in pivotal transformations that are redefining the disposable medical device landscape. Cutting-edge materials science has paved the way for lightweight, biocompatible polymers that reduce environmental footprint while maintaining clinical performance. At the same time, digital integration has enabled smart devices with embedded sensors and connectivity features, granting clinicians unprecedented real-time insights into patient status.Concurrently, regulatory frameworks are evolving to address emerging risks and promote quality assurance. New guidelines on material traceability and post-market surveillance are creating higher compliance thresholds, compelling manufacturers to enhance transparency and adopt advanced quality management systems. In parallel, reimbursement policies in key markets are adjusting to incentivize innovative solutions that demonstrate clear clinical and economic value.
In addition to regulatory and technological shifts, sustainability initiatives have taken center stage within corporate strategies. Industry leaders are exploring closed-loop sterilization programs and recyclable packaging designs as part of broader environmental commitments. Together, these transformative dynamics are catalyzing new business models and strategic partnerships, laying the foundation for sustainable growth and differentiated market positioning.
Assessing the Complex Implications of 2025 U.S. Tariff Policies on Global Supply Chains and Cost Structures within the Disposable Medical Device Sector
As 2025 approaches, the reshaping of United States tariff policies has triggered significant reverberations across the global supply chain for disposable medical devices. The introduction of additional duties on certain raw materials and finished components has elevated input costs and prompted manufacturers to reassess sourcing strategies. In response, many suppliers are negotiating alternative trade agreements or collaborating with domestic partners to mitigate financial impacts and ensure continuity of supply.Moreover, the evolving tariff landscape has influenced procurement decisions at provider institutions. Hospitals and clinics are increasingly seeking fixed-cost contracts and long-term agreements to insulate procurement budgets from sudden price fluctuations. At the same time, strategic stockpiling and nearshoring initiatives have gained traction as risk management measures, although these approaches require careful balance to avoid obsolescence and storage constraints.
To sustain innovation pipelines amidst elevated costs, many firms are extending collaboration frameworks with contract research and manufacturing partners. These alliances enable cost-sharing and rapid scalability, ensuring that novel product introductions remain viable despite heightened tariff pressures. Furthermore, the drive toward automation in manufacturing processes is gaining momentum, as capital investments in advanced production lines can offset incremental duty expenses over time.
In light of these complexities, financial forecasting and scenario planning have become critical organizational capabilities. Companies that proactively model tariff adjustments and associated logistics expenses are better positioned to develop agile pricing strategies. This adaptability not only preserves profit margins but also fortifies relationships with key stakeholders by demonstrating a commitment to operational resilience in a rapidly changing trade environment.
Decoding Critical Segment Performance across Device Categories, Materials, End Users, and Applications in the Disposable Medical Device Market
Understanding the disposable medical device market requires a nuanced examination of device categories, each defined by distinct clinical functions and material compositions. Within the catheter segment, indwelling variants cater to long-term vascular access while intermittent options serve rapid fluid delivery in ambulatory settings. Similarly, disposable masks encompass both high-filtration respirators such as N95 models and conventional surgical face coverings, addressing variable infection control requirements across healthcare environments. Surgical gloves present a trifurcated landscape of latex, nitrile, and vinyl alternatives, each chosen for tactile sensitivity, chemical resistance, or cost considerations. In the syringe space, pre-filled systems offer accuracy and convenience, safety-engineered devices minimize needlestick risks, and single-use plastic designs deliver straightforward disposability for routine injections.Material selection further influences performance characteristics and regulatory classification. Non-woven fabric remains a cornerstone for barrier protection, while polyethylene and PVC provide structural integrity for molded components. Rubber continues to enable flexible seals and tubing in critical applications. Across end users, ambulatory surgical centers are driving demand for portable solutions, clinics prioritize cost-effective consumables, home healthcare agencies value ease of use, and hospitals necessitate scalable inventories to support high-volume procedures. In parallel, application areas span cleaning and disinfection protocols, injectable therapies, respiratory support interventions, and wound care management, each presenting unique design and regulatory challenges.
Through this multilayered segmentation framework, industry stakeholders can align product innovation and marketing strategies with clinical needs, regulatory pathways, and consumption patterns that vary across use cases.
Exploring Distinct Growth Drivers and Market Dynamics across the Americas, Europe Middle East & Africa, and Asia-Pacific Regions
Regional dynamics exert a profound influence on the disposable medical device market, with each geography characterized by distinct drivers and market conditions. In the Americas, advanced healthcare infrastructure and a strong emphasis on procedural safety have accelerated the adoption of single-use technologies in hospital networks and outpatient surgery centers. Moreover, policy measures targeting infection control, coupled with reimbursement frameworks that reward innovative care delivery, are sustaining high consumption rates of sterile disposables.Meanwhile, the Europe, Middle East & Africa region presents a heterogeneous landscape shaped by regulatory convergence and variable economic development. The European Union's Medical Device Regulation has elevated quality standards, prompting suppliers to invest in enhanced documentation and post-market surveillance capabilities. In contrast, emerging markets across the Middle East and Africa are experiencing growing demand for basic consumables as healthcare access expands, even as cost sensitivity remains a key consideration for procurement agencies.
Across the Asia-Pacific region, demographic shifts and rapid urbanization drive expansion of both public and private healthcare facilities. In countries such as China and India, government initiatives to improve rural healthcare infrastructure are creating significant opportunities for cost-effective disposable solutions. Additionally, a burgeoning domestic manufacturing base is strengthening regional supply resilience and enabling tailored product designs that address local clinical requirements.
Highlighting Competitive Strategies, Innovation Trajectories, and Partnership Models among Leading Disposable Medical Device Companies
Leading companies in the disposable medical device arena are distinguished by their capacity to blend operational excellence with innovation-driven growth strategies. Global medtech conglomerates leverage extensive research and development budgets to introduce advanced materials and smart device platforms, while forging strategic partnerships to accelerate commercialization. In parallel, regional manufacturers specialize in niche segments, capitalizing on localized production efficiencies and deep customer relationships to capture market share in price-sensitive environments.Collaborative models have become increasingly prevalent, with alliances across contract research organizations, healthcare providers, and academic institutions facilitating co-development of next-generation consumables. Such partnerships not only diversify risk but also enrich the innovation pipeline with practical clinical insights. Moreover, merger and acquisition activity remains robust, as established players seek to augment their product portfolios and gain entry into high-growth geographies.
In this competitive landscape, agility in regulatory compliance and supply chain management emerges as a critical differentiator. Companies that proactively anticipate standard revisions and invest in digital platforms for traceability and quality control are better positioned to navigate complex market entry requirements. As a result, a dual focus on strategic expansion and operational resilience underpins the success of leading industry participants.
Formulating Strategic Initiatives and Operational Best Practices to Strengthen Market Position and Drive Sustainable Growth in Disposable Devices
To thrive in the evolving disposable medical device arena, industry leaders must adopt a proactive posture that balances innovation with operational rigor. First, integrating sustainable material solutions into product design will not only address environmental imperatives but also resonate with value-driven purchasers. Concurrently, advancing digital capabilities-such as embedding sensor technology for usage tracking and outcomes measurement-can unlock new revenue models and strengthen customer engagement.Furthermore, diversifying the supply base by identifying strategic nearshoring partners can mitigate tariff-related disruptions and enhance responsiveness to market fluctuations. Engaging in long-term procurement agreements with key healthcare networks will secure stable demand while fostering collaborative cost-management initiatives. In tandem, forging alliances with academic and clinical research centers can expedite product validation and reinforce evidence-based value propositions.
Additionally, maintaining rigorous regulatory intelligence and investing in automated quality management systems will streamline compliance processes and accelerate time to market. Finally, cultivating talent through specialized training programs in areas such as regulatory affairs and digital manufacturing will ensure workforce readiness for future challenges. By implementing these strategic initiatives, companies can reinforce competitive positioning and sustain profitable growth in a dynamic global marketplace.
Outlining a Rigorous Research Framework Combining Primary Interviews, Secondary Analysis, and Data Validation for Comprehensive Market Insights
The research methodology underpinning this analysis combines a structured framework of primary and secondary activities designed to ensure depth, accuracy, and reliability. Primary research entailed in-depth interviews with senior executives, regulatory specialists, and procurement directors across healthcare settings. These conversations yielded qualitative insights into strategic priorities, supply chain dynamics, and regulatory compliance challenges shaping the disposable medical device sector.Secondary research encompassed a comprehensive review of publicly available documents, including regulatory filings, patent databases, and industry publications. This phase validated primary findings and provided a robust context for interpreting market developments. Where discrepancies emerged, data triangulation techniques were employed to reconcile divergent viewpoints and secure consensus-driven conclusions. Throughout the process, a clear emphasis was placed on sourcing information from reputable government agencies, academic journals, and professional associations to uphold methodological integrity.
Quantitative data points were subject to rigorous quality checks, including cross-referencing against multiple independent sources and applying statistical consistency tests. The final deliverable reflects a synthesis of qualitative perspectives and empirical evidence, presented in a format that supports actionable decision-making. This methodological rigor ensures that the insights offered here can serve as a reliable foundation for strategic planning and investment decisions.
Synthesizing Core Findings and Strategic Considerations to Guide Decision Makers in the Evolving Disposable Medical Device Environment
As the disposable medical device industry enters a new phase of strategic evolution, core themes emerge around innovation, regulatory resilience, and supply chain optimization. Technological advancements in materials science and digital integration are poised to redefine product differentiation, while regional market dynamics will continue to shape demand patterns. The impact of evolving tariff policies underscores the importance of flexible sourcing strategies and agile cost-management approaches.Segmentation analysis highlights the diversity of clinical applications and end-user requirements, underscoring the need for tailored solutions that address specific procedural contexts. Meanwhile, leading companies that balance growth ambitions with operational discipline are setting the standard for competitive performance. The recommendations outlined herein offer a roadmap for industry stakeholders to navigate complexity and capitalize on emerging opportunities.
In sum, this executive summary distills critical insights and strategic imperatives that will guide decision makers as they chart the future of disposable medical devices, ensuring both clinical impact and commercial success.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Catheters
- Diagnostic Test Kits
- IV Sets & Infusion Devices
- Respiratory Devices
- Surgical Gloves
- Surgical Instruments
- Scalpels
- Scissors
- Syringes & Needles
- Material Type
- Latex
- Polyethylene
- Polypropylene
- PVC
- Application
- Cleaning & Disinfection
- Injections
- Respiratory Therapy
- Wound Care
- End User
- Ambulatory Surgical Centers
- Clinics
- Home Healthcare
- Hospitals
- Distribution Channel
- Offline Retail
- Online Retail
- Company Websites
- E-Commerce Platforms
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- 3M Company
- Ambu A/S
- Ansell Limited
- B. Braun SE
- Baxter International Inc.
- Becton, Dickinson and Company
- Cardinal Health, Inc.
- Coloplast A/S
- GPC Medical Ltd.
- Hartalega Holdings Berhad
- IndoSurgicals Private Limited
- Kimberly-Clark Corporation
- McKesson Corporation
- Medline Industries, L.P.
- Medtronic plc
- Mercator Medical S.A.
- Mölnlycke Health Care AB
- Nipro Corporation
- Owens & Minor, Inc.
- Puritan Medical Products Co., LLC
- Semperit AG Holding
- Smith & Nephew PLC
- Terumo Corporation
- Top Glove Corporation Bhd
- UFP Technologies, Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Disposable Medical Device market report include:- 3M Company
- Ambu A/S
- Ansell Limited
- B. Braun SE
- Baxter International Inc.
- Becton, Dickinson and Company
- Cardinal Health, Inc.
- Coloplast A/S
- GPC Medical Ltd.
- Hartalega Holdings Berhad
- IndoSurgicals Private Limited
- Kimberly-Clark Corporation
- McKesson Corporation
- Medline Industries, L.P.
- Medtronic plc
- Mercator Medical S.A.
- Mölnlycke Health Care AB
- Nipro Corporation
- Owens & Minor, Inc.
- Puritan Medical Products Co., LLC
- Semperit AG Holding
- Smith & Nephew PLC
- Terumo Corporation
- Top Glove Corporation Bhd
- UFP Technologies, Inc.
Table Information
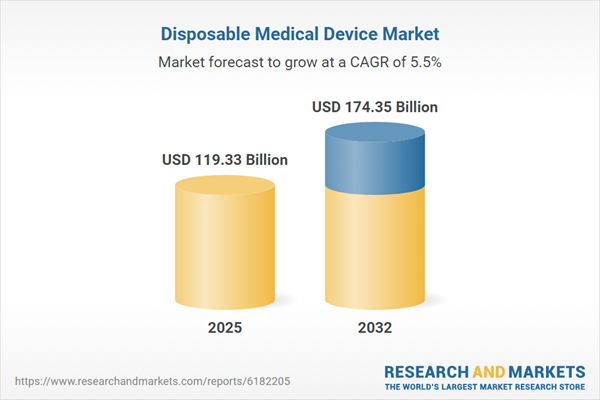
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 119.33 Billion |
| Forecasted Market Value ( USD | $ 174.35 Billion |
| Compound Annual Growth Rate | 5.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |