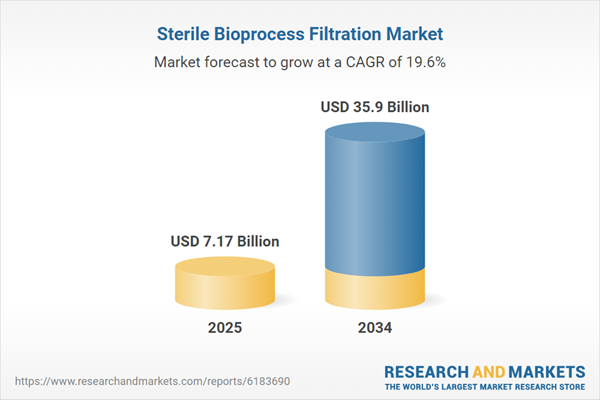
Sterile Bioprocess Filtration Market
The Sterile Bioprocess Filtration market spans depth and membrane filters, single-use assemblies (tubing, connectors, capsules), sterile bulk and final-fill filters, virus filters and prefilters, vent/air filters, tangential-flow filtration (TFF) cassettes/hollow fibers, integrity testing instruments, and turnkey skids with automation and analytics. Core end-uses include monoclonal antibodies and recombinant proteins, vaccines (viral vectors, subunit, mRNA/LNP), cell and gene therapies, plasmids, and advanced microbial/enzymatic processes. Momentum is shaped by platformed, single-use downstream trains; high-flux, low-fouling membranes for viscous feeds; intensified upstream titers driving higher particulate/bioburden loads; and integrated, gamma-ready flow paths that shorten changeover and deviation risk. Growth drivers include the shift to multi-product facilities, rapid scale-out for CGT, resilient supply strategies, and regulatory focus on sterility assurance and data integrity. Competitive dynamics feature diversified bioprocess majors and specialist innovators competing on throughput at target LRV, robustness to process variability, validated extractables/leachables, and end-to-end design support. Differentiation increasingly rests on virus filtration performance with reliable prefiltration, real-time integrity testing, automated skid control (alarms/interlocks, audit trails), and standardized assemblies that compress qualification. Headwinds include membrane supply constraints, gamma irradiation capacity bottlenecks, higher upstream impurities challenging flux, and the need to reconcile speed-to-clinic with lifecycle cost. Overall, the market is moving from component purchasing to platformed, specification-driven solutions - filter media + hardware + automation + QA documentation - delivering reproducible sterility assurance, faster batch release, and resilient operations from clinical to commercial scale.Sterile Bioprocess Filtration Market Key Insights
- Platformed single-use trains win scale-outs: Standard capsule/connector libraries, pre-validated BOMs, and electronic batch records reduce engineering time and deviation risk across multi-suite facilities and CMOs.
- Virus filtration is the bottleneck to beat: Next-gen PES/PVDF and composite media with smart prefiltration strategies sustain flux and LRV under high-protein, high-excipient feeds - minimizing costly retests and holds.
- Upstream intensity reshapes sizing: Higher cell densities and titers increase colloids and host cell impurities; graded depth + membrane stacks and inline conditioning stabilize pH/conductivity to protect final filters.
- Integrity by design, not just at release: Automated forward flow/bubble point tests, leak-before-break housings, and inline monitors enable in-process assurance and real-time deviation detection.
- Automation and data integrity matter: 21 CFR Part 11/Annex 11-ready skids with closed-loop pressure/flow control, interlocks, and audit trails standardize outcomes and speed investigations.
- Extractables/leachables confidence is table stakes: Gamma-stable polymers with comprehensive E/L packages and compatibility matrices accelerate qualification across mAbs, rAAV, and LNP processes.
- TFF shifts toward intensified formats: High-area cassettes and hollow fibers with low protein binding support continuous or semi-continuous operations; smarter CIP/SIP or single-use swaps cut downtime.
- Final fill/finish gets smarter and smaller: Sterile filters paired with low-shear pumps, isolators/RABS, and closed paths reduce microbial ingress and particulate risk, supporting smaller batch sizes for CGT.
- Supply resilience is strategic: Dual-qualified media, regional assembly, inventory pooling, and modular designs mitigate shortages and irradiation queue times, protecting campaign schedules.
- Sustainability enters specs: Lower hold-up volume, recyclable housings, and solvent/energy-lean sterilization strategies reduce footprint without sacrificing sterility assurance or throughput.
Sterile Bioprocess Filtration Market Reginal Analysis
North America
Biopharma concentration, CGT scale-out, and strong CMO capacity anchor demand for standardized single-use assemblies, high-performance virus filters, and automated integrity testing. Buyers prioritize platform compatibility across sites, rapid tech transfer, and robust E/L documentation. Resilience planning - dual suppliers, regional assembly, and irradiation slots - features in contracts. Skid automation with compliant data trails and remote support shortens investigations and batch release.Europe
Regulatory rigor and cross-site harmonization drive adoption of validated filtration trains with thorough extractables data and lifecycle risk assessments. Continuous and intensified downstream pilots expand, elevating TFF and closed-system requirements. Sustainability expectations favor low hold-up designs and reparable housings where appropriate. Tenders weigh sterility assurance, cybersecurity of control systems, and supplier quality systems alongside total cost of ownership.Asia-Pacific
Rapid capacity build-out in vaccines, biosimilars, and CGT fuels demand for cost-efficient, gamma-ready single-use lines and scalable virus filtration. Local manufacturing of assemblies grows, while premium centers adopt advanced automation and in-line integrity testing. Buyers emphasize price-performance, technical training, and fast service. Standard libraries and pre-engineered skids reduce start-up times across expanding facilities.Middle East & Africa
New biopharma hubs and fill-finish projects seek turnkey, closed, and easily validated sterile filtration trains. Procurement focuses on robustness, operator training, and vendor support for QA documentation and qualification. Environmental constraints elevate interest in low-hold-up, low-waste designs. Partnerships with global suppliers cover technology transfer and stocking strategies to buffer logistics variability.South & Central America
Emerging vaccine and biologics programs prioritize standardized, single-use filtration with strong supplier onboarding and training. Currency and import factors heighten attention to lifecycle cost, local service, and spare-assembly availability. Facilities adopt modular skids and platform BOMs for faster validation. Suppliers offering comprehensive E/L packages, remote troubleshooting, and dual-source strategies gain preference in public and private tenders.Sterile Bioprocess Filtration Market Segmentation
By Product
- Membrane Filters
- Depth Filters
- Cartridge Filters
- Capsule Filters
- Filtration Accessories
- Others
By Workflow
- Upstream
- Downstream
- Fermentation
- Aseptic Filling
By Material
- Polyethersulfone (PES)
- Polyvinylidene Fluoride (PVDF)
- Polytetrafluoroethylene (PTFE)
- Nylon
- Others
By End-User
- Academic & Research Institutes
- Biopharmaceutical & Biotechnology Companies
- CMOs & CROs
Key Market players
Sartorius Stedim Biotech, Merck KGaA (MilliporeSigma), Pall Corporation (Danaher), Cytiva (Danaher), 3M (3M Purification), Parker Hannifin (Parker Bioscience Filtration), Thermo Fisher Scientific, Meissner Filtration Products, Asahi Kasei Medical (Planova), Repligen Corporation, Saint-Gobain Life Sciences, Cobetter Filtration Group, Porvair Filtration Group, W. L. Gore & Associates, Donaldson Company, Inc.Sterile Bioprocess Filtration Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Sterile Bioprocess Filtration Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Sterile Bioprocess Filtration market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Sterile Bioprocess Filtration market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Sterile Bioprocess Filtration market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Sterile Bioprocess Filtration market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Sterile Bioprocess Filtration market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Sterile Bioprocess Filtration value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Sterile Bioprocess Filtration industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Sterile Bioprocess Filtration Market Report
- Global Sterile Bioprocess Filtration market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Sterile Bioprocess Filtration trade, costs, and supply chains
- Sterile Bioprocess Filtration market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Sterile Bioprocess Filtration market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Sterile Bioprocess Filtration market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Sterile Bioprocess Filtration supply chain analysis
- Sterile Bioprocess Filtration trade analysis, Sterile Bioprocess Filtration market price analysis, and Sterile Bioprocess Filtration supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Sterile Bioprocess Filtration market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Sartorius Stedim Biotech
- Merck KGaA (MilliporeSigma)
- Pall Corporation (Danaher)
- Cytiva (Danaher)
- 3M (3M Purification)
- Parker Hannifin (Parker Bioscience Filtration)
- Thermo Fisher Scientific
- Meissner Filtration Products
- Asahi Kasei Medical (Planova)
- Repligen Corporation
- Saint-Gobain Life Sciences
- Cobetter Filtration Group
- Porvair Filtration Group
- W. L. Gore & Associates
- Donaldson Company Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 7.17 Billion |
| Forecasted Market Value ( USD | $ 35.9 Billion |
| Compound Annual Growth Rate | 19.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |