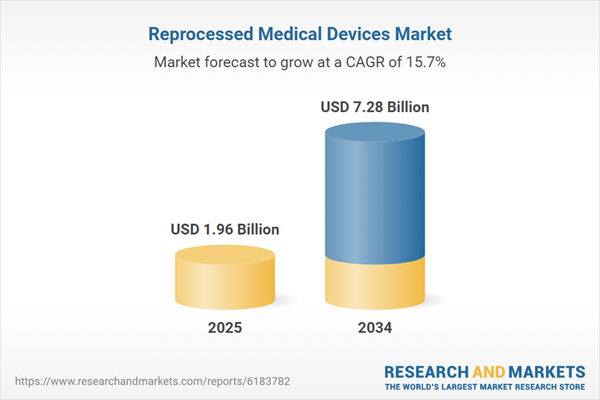
Reprocessed Medical Devices Market
The reprocessed medical devices market covers validated, cleaned, tested, and sterilized versions of select single-use and reusable devices that meet equivalent safety and performance to new items. Hospital priorities - cost containment, supply resilience, and waste reduction - are pushing adoption across electrophysiology (EP) diagnostic catheters, compression sleeves, pulse oximeter probes, laparoscopic instruments, external fixation components, and operating-room disposables. Latest trends include expansion of UDI-enabled traceability, digital QA dashboards, and life-cycle accounting that quantifies carbon and waste savings alongside budget impact. Clinical engineering teams increasingly partner with third-party FDA/CE-compliant reprocessors to standardize collections, set evidence thresholds, and align reprocessed SKUs to procedure packs. Drivers span inflationary pressure on procurement, sustainability goals, and broader acceptance of risk-managed circular models after pandemic-era supply shocks. Competitive dynamics pit OEM “new-only” strategies against hybrid models and specialized reprocessors offering validated reprocessing cycles, lot-level documentation, and shared-savings contracts. Differentiation centers on robust decontamination (manual + automated), functional testing beyond sterility (electrical integrity, lumen patency, torque/bend), and sterilization modality fit (ethylene oxide, low-temperature hydrogen peroxide plasma, steam for select items). Hospital committees scrutinize clinical equivalence, turnaround times, and liability cover; payers and group purchasing organizations increasingly recognize reprocessed alternatives within formularies and bundles. Looking forward, expect broader inclusion of sensorized devices, analytics-guided recapture programs, and MDR-aligned technical files that ease cross-border adoption. As ESG reporting tightens, validated reprocessing that delivers measurable savings without compromising outcomes will move from opportunistic to programmatic within integrated delivery networks and private hospital chains.Reprocessed Medical Devices Market Key Insights
- Value creation beyond price cuts
- Regulatory clarity drives confidence
- Category expansion led by EP and OR disposables
- Quality by design: beyond sterility
- Traceability and UDI integration
- Sterilization modality fit matters
- ESG and circular procurement
- OEM-reprocessor coexistence
- Change management and clinician trust
- Data-enabled program governance
Reprocessed Medical Devices Market Reginal Analysis
North America
Established regulatory pathways and large integrated delivery networks support scaled programs in cardiology, OR, and med-surg. GPO frameworks normalize reprocessed SKUs in contracts, while ESG reporting amplifies waste-reduction benefits. Competitive intensity is high among specialized reprocessors, with hospital systems seeking multi-year, KPI-linked agreements and robust field education.Europe
Adoption aligns with country-specific interpretations under MDR, producing a patchwork of practices. University hospitals lead pilots in EP and OR disposables, emphasizing rigorous technical files and clinical committee oversight. Sustainability mandates in public procurement encourage trials, but cross-border harmonization and liability allocation remain active discussion points.Asia-Pacific
Diverse regulatory maturity yields varied uptake: advanced markets pilot tightly controlled programs with tertiary hospitals, while others focus on reusables. Cost pressure and urban hospital consolidation create interest, especially in private chains. Local sterilization capacity, workforce training, and OEM stance significantly influence feasibility.Middle East & Africa
Private hospital groups and flagship public facilities explore reprocessing to manage costs and strengthen supply resilience. Program scale is constrained by regulatory clarity and availability of accredited partners. Training, documentation, and insurer engagement are prerequisites for broader rollout, with early focus on low-risk categories.South & Central America
Macroeconomic volatility and currency pressure heighten interest in reprocessed options within cardiology and OR. Regulatory acceptance varies, prompting hospital-level pilots with tight QA oversight. Reliable logistics, service contracts, and clinician education determine momentum, especially within growing private networks.Reprocessed Medical Devices Market Segmentation
By Product
- Cardiovascular
- Laparoscopic
- Gastroenterology
- General Surgery Devices
- Orthopedic Devices
By Type
- Third-party Reprocessing
- In-house Reprocessing
By End-user
- Hospitals
- Home Healthcare
- Others
Key Market players
Stryker Sustainability Solutions, Sterilmed (Johnson & Johnson), Medline ReNewal, Cardinal Health Sustainable Technologies, Innovative Health, Hygia Health Services, Vanguard AG, ReNu Medical, Northeast Scientific (NEScientific), SureTek Medical, The ReMed Group (TRG), SteriPro, DeRoyal Reprocessing, Avante Health Solutions, Arjo.Reprocessed Medical Devices Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Reprocessed Medical Devices Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Reprocessed Medical Devices market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Reprocessed Medical Devices market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Reprocessed Medical Devices market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Reprocessed Medical Devices market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Reprocessed Medical Devices market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Reprocessed Medical Devices value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Reprocessed Medical Devices industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Reprocessed Medical Devices Market Report
- Global Reprocessed Medical Devices market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Reprocessed Medical Devices trade, costs, and supply chains
- Reprocessed Medical Devices market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Reprocessed Medical Devices market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Reprocessed Medical Devices market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Reprocessed Medical Devices supply chain analysis
- Reprocessed Medical Devices trade analysis, Reprocessed Medical Devices market price analysis, and Reprocessed Medical Devices supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Reprocessed Medical Devices market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Stryker Sustainability Solutions
- Sterilmed (Johnson & Johnson)
- Medline ReNewal
- Cardinal Health Sustainable Technologies
- Innovative Health
- Hygia Health Services
- Vanguard AG
- ReNu Medical
- Northeast Scientific (NEScientific)
- SureTek Medical
- The ReMed Group (TRG)
- SteriPro
- DeRoyal Reprocessing
- Avante Health Solutions
- Arjo.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 1.96 Billion |
| Forecasted Market Value ( USD | $ 7.28 Billion |
| Compound Annual Growth Rate | 15.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |