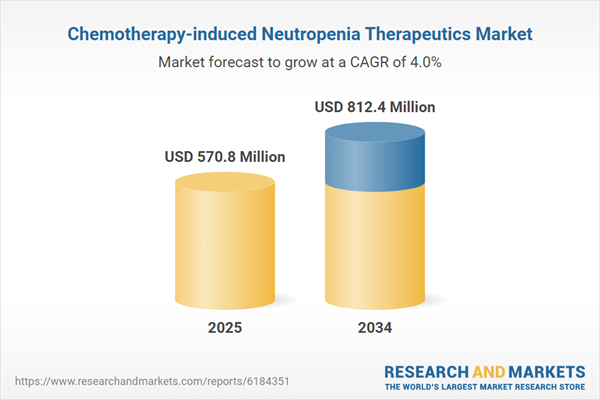
Chemotherapy-induced Neutropenia Therapeutics Market
The Chemotherapy-induced Neutropenia Therapeutics Market is transitioning from reactive rescue to proactive, risk-managed care pathways as oncology pivots to preserve dose intensity, prevent infection, and shift services outpatient. Demand is anchored by prophylactic and on-demand granulocyte colony-stimulating factors (G-CSFs), supported by biosimilar adoption, antimicrobial stewardship, and standardized febrile-neutropenia bundles. Innovation focuses on long-acting and on-body G-CSF delivery, digital reminders, and risk-adapted protocols that align with guideline triggers and patient preference for home care. Decision makers prioritize regimens that minimize breakthrough neutropenia, lower hospitalization and emergency visits, and maintain chemotherapy schedules, while balancing thrombosis, bone pain, and cost. Biosimilar competition intensifies value analysis and expands access, yet originators differentiate with device usability, patient support, and real-world outcomes. Comorbidity profiling, prior cycle nadirs, and regimen intensity inform prophylaxis; integrated pathways blend G-CSF with oral antibiotics for select high-risk cohorts under antimicrobial stewardship. Pharmacy and therapeutics committees evaluate total-episode economics, including infusion chair time, caregiver burden, and readmission risk, pushing preference lists toward predictable, low-touch options. Outreach nursing, specialty pharmacy, and home infusion expand adherence and monitoring, while EMR prompts and analytics standardize risk scoring and follow-up labs. In parallel, pipeline work explores novel neutrophil-sparing regimens, cytokine engineering, and adjuncts that modulate marrow recovery without compromising antitumor efficacy. Overall, the market is professionalizing around outcomes-anchored, patient-centric models that emphasize reliability, convenience, and stewardship, positioning CIN therapeutics as a foundational enabler of modern systemic cancer care across tumor types and geographies. Vendor services, education, and inventory agility further de-risk cycles and sustain continuity goals.Chemotherapy-induced Neutropenia Therapeutics Market Key Insights
- Evidence-driven prophylaxis. Risk tools combining regimen intensity, prior nadirs, and comorbidities are standardizing primary prophylaxis, reducing dose delays and unplanned acute care while aligning with stewardship objectives across tumor types.
- Long-acting and on-body growth. Extended-duration and wearable doses improve adherence and convenience, supporting weekend discharges and home care models without additional clinic visits or complex scheduling logistics.
- Biosimilar expansion with guardrails. Broader access and budget headroom accelerate use, while institutions maintain interchangeability governance, device training, and pharmacovigilance to protect outcomes and minimize variability.
- Total-episode economics dominate. Value analysis weighs device cost against avoided admissions, protected dose intensity, and chair-time relief - favoring predictable, low-touch options with robust real-world performance.
- Safety and tolerability focus. Programs address bone pain, injection-site reactions, and rare thrombotic signals via dosing optimization, supportive co-medications, and patient education to sustain adherence.
- Digital and EMR integration. Order sets, discharge prompts, and remote check-ins close care gaps, trigger timely dosing, and streamline follow-up labs following high-risk cycles.
- Home-based care momentum. Specialty pharmacy, visiting nurses, and self-administration pathways extend reach outside infusion centers, improving patient experience and smoothing operational peaks.
- Antimicrobial stewardship alignment. Select high-risk cohorts combine G-CSF with targeted oral antibiotics under protocols that preserve microbiome balance and mitigate resistance pressure.
- Pipeline diversification. Next-gen cytokines, neutrophil-sparing regimens, and adjunctive marrow modulators aim to shorten nadirs and improve quality of life without undermining anticancer efficacy.
- Training and service as differentiators. Vendor education, device usability, inventory agility, and transition-of-care support increasingly decide formulary placement in competitive bids.
Chemotherapy-induced Neutropenia Therapeutics Market Reginal Analysis
North America
Integrated delivery networks emphasize standardized risk assessment, EMR-embedded order sets, and on-body dosing to support early discharge and reduce emergency visits. Biosimilar uptake is strong under enterprise contracts, with device familiarity and patient-support programs influencing brand preference. Home-based administration through specialty pharmacy grows alongside remote monitoring. Value committees scrutinize total-episode outcomes, pushing for predictable prophylaxis that protects chemotherapy cadence. Education addresses management of bone pain and rare adverse events to sustain adherence, while payer policies align around consistent pathways and documented real-world performance.Europe
Guideline discipline and tendering frameworks drive consistent prophylaxis strategies, with biosimilars broadly embedded under interchangeability governance. Hospitals favor long-acting and clinic-light regimens that fit day-case oncology. Antimicrobial stewardship is tightly integrated, reserving combined approaches for clearly defined high-risk profiles. Health-technology assessments prioritize quality-of-life indicators and avoided admissions. Cross-border procurement encourages harmonized training and device handling, while registries capture safety and effectiveness signals to refine national pathways. Environmental and medication-waste considerations influence device and packaging choices in formulary reviews.Asia-Pacific
Heterogeneous systems blend rapid adoption in developed markets with access-building initiatives elsewhere. Major centers prioritize long-acting or on-body options that reduce follow-up visits and congestion in high-throughput clinics. Education focuses on risk scoring, consistent timing post-chemotherapy, and recognizing early infection signs. Domestic biosimilar ecosystems expand access, with local manufacturing supporting cost containment. Partnerships with home-care providers and digital adherence tools evolve quickly, particularly in urban hubs. Public programs increasingly embed standardized CIN pathways to protect chemotherapy intensity across overcrowded services.Middle East & Africa
Growth tracks investments in comprehensive cancer centers and national oncology networks. Institutions prioritize simple, reliable prophylaxis with strong usability and minimal monitoring needs, supporting constrained outpatient capacity. Procurement emphasizes predictable supply, training, and vendor service to ensure seamless transitions across inpatient and ambulatory settings. Awareness programs educate patients on self-administration and red flags for febrile events. Gradual expansion of specialty pharmacy and home nursing bolsters adherence, while centralized protocols and stewardship measures guide antibiotic co-use in high-risk cohorts.South & Central America
Urban tertiary centers lead adoption with protocolized prophylaxis to prevent dose reductions and unplanned admissions. Biosimilars widen access under budget pressures; formulary decisions weigh device handling, patient training, and logistics across public and private payers. Outreach nursing, pharmacy counseling, and SMS reminders support timely dosing in dispersed geographies. Institutions pursue bundled-care contracts that link drug supply with education and pharmacovigilance. Regional data collection and participation in multinational registries strengthen confidence and drive incremental coverage by health systems.Chemotherapy-induced Neutropenia Therapeutics Market Segmentation
By Type
- Antibiotic Therapy
- Granulocyte Colony-Stimulating Factor Therapy
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Others
Key Market players
Amgen, Sandoz, Teva Pharmaceutical Industries, Pfizer (Hospira), Coherus BioSciences, Biocon Biologics, Viatris, Fresenius Kabi, Amneal Pharmaceuticals, Dr. Reddy’s Laboratories, Intas Pharmaceuticals (Accord Healthcare), Apotex, Lupin, Mundipharma, Assertio TherapeuticsChemotherapy-induced Neutropenia Therapeutics Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Chemotherapy-induced Neutropenia Therapeutics Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Chemotherapy-induced Neutropenia Therapeutics market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Chemotherapy-induced Neutropenia Therapeutics market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Chemotherapy-induced Neutropenia Therapeutics market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Chemotherapy-induced Neutropenia Therapeutics market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Chemotherapy-induced Neutropenia Therapeutics market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Chemotherapy-induced Neutropenia Therapeutics value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Chemotherapy-induced Neutropenia Therapeutics industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Chemotherapy-induced Neutropenia Therapeutics Market Report
- Global Chemotherapy-induced Neutropenia Therapeutics market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Chemotherapy-induced Neutropenia Therapeutics trade, costs, and supply chains
- Chemotherapy-induced Neutropenia Therapeutics market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Chemotherapy-induced Neutropenia Therapeutics market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Chemotherapy-induced Neutropenia Therapeutics market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Chemotherapy-induced Neutropenia Therapeutics supply chain analysis
- Chemotherapy-induced Neutropenia Therapeutics trade analysis, Chemotherapy-induced Neutropenia Therapeutics market price analysis, and Chemotherapy-induced Neutropenia Therapeutics supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Chemotherapy-induced Neutropenia Therapeutics market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Amgen
- Sandoz
- Teva Pharmaceutical Industries
- Pfizer (Hospira)
- Coherus BioSciences
- Biocon Biologics
- Viatris
- Fresenius Kabi
- Amneal Pharmaceuticals
- Dr. Reddy’s Laboratories
- Intas Pharmaceuticals (Accord Healthcare)
- Apotex
- Lupin
- Mundipharma
- Assertio Therapeutics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 570.8 Million |
| Forecasted Market Value ( USD | $ 812.4 Million |
| Compound Annual Growth Rate | 4.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |