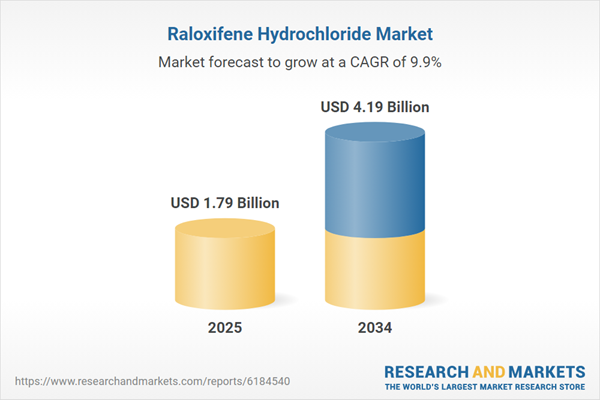
Raloxifene Hydrochloride Market
Raloxifene hydrochloride is a selective estrogen receptor modulator (SERM) indicated primarily for prevention and treatment of postmenopausal osteoporosis and for reducing the risk of invasive breast cancer in select populations. The market has transitioned from a single innovator brand to a broad-based, multi-generic landscape anchored by 60 mg film-coated tablets, with value pools split between reimbursed retail scripts, hospital procurement, and tenders in public systems. Top end-uses concentrate on long-term bone health management in postmenopausal women, second-line therapy where oral bisphosphonates are unsuitable, and risk-reduction protocols in high-risk cohorts. Key trends include formulary shifts toward cost-effective generics, real-world evidence informing persistence and safety monitoring, and heightened quality requirements on API/finished dose manufacturing (DMFs/CEPs, QbD, nitrosamine risk assessments, and continuous improvement in impurity controls). Demand is lifted by aging demographics, fracture-prevention policies, and expanding screening (e.g., FRAX-based risk stratification), while moderated by competition from bisphosphonates, denosumab, HRT options, and other SERMs. The competitive landscape includes API producers with backward integration, global and regional generic FDF manufacturers, and CDMOs offering tech transfers and site redundancy. Differentiation centers on proven bioequivalence, clean impurity profiles, robust pharmacovigilance, patient-support and adherence programs, and supply reliability for tender markets. Portfolio extensions - such as vitamin D co-formulations and pack sizes aligned to adherence cycles - support brand stickiness without altering the core clinical positioning. Overall, growth depends on disciplined price-volume management, access strategies in emerging markets, regulatory compliance across jurisdictions, and lifecycle stewardship that balances safety communication with measurable outcomes in fracture and risk-reduction pathways.Raloxifene Hydrochloride Market Key Insights
- Post-LOE market structure
- Indication focus and patient selection
- Safety profile stewardship
- API security and quality
- Manufacturing excellence
- Access and reimbursement
- Real-world evidence and adherence
- Portfolio and packaging strategy
- Digital and HCP engagement
- Emerging-market optionality
Raloxifene Hydrochloride Market Reginal Analysis
North America
The market is mature and highly genericized, with payers enforcing step therapy and reference prices. Osteoporosis management pathways emphasize risk stratification and shared decision-making, supporting use in patients unsuitable for first-line alternatives. Pharmacovigilance expectations and REMS-style communication drive consistent safety messaging. E-pharmacy channels aid refill adherence, while wholesaler service levels and GPO contracts influence share stability.Europe
Stringent GMP and pharmacovigilance frameworks favor suppliers with CEPs, reliable batch documentation, and uninterrupted deliveries. National tenders and reference pricing shape margins, but stable demand flows from guideline-driven osteoporosis care and risk-reduction protocols. Hospital and community pharmacy segments both matter, with parallel-trade resilience a differentiator. Sustainability and supply-chain transparency increasingly enter procurement scoring.Asia-Pacific
Volume growth is underpinned by aging populations, rising DEXA access, and expanding reimbursement in middle-income markets. Local manufacturers compete with global generics; success depends on dossier quality, bioequivalence performance, and price points tailored to public and private channels. Hot-humid stability and patient education programs are critical to persistence. E-commerce and specialty retail broaden reach in urban centers.Middle East & Africa
Market development follows improvements in osteoporosis screening and formulary inclusion within public systems. Procurement relies on tenders and distributor networks; consistent supply, Arabic/Local language labeling, and pharmacovigilance readiness are key. Private hospitals in the Gulf adopt evidence-based protocols faster, while broader access progresses with clinician education and budget expansion. Cold-chain is not required, but warehouse QA and humidity control are important.South & Central America
Economic cycles and currency volatility shape pricing and inventory strategies. Public tenders coexist with private retail channels, making dual-route supply essential. Local fill-finish or tech-transfer partnerships can offset import duties and lead times. Physician education and patient-support programs improve adherence amid competing therapies. Regulatory convergence and post-marketing surveillance strengthen confidence across major markets.Raloxifene Hydrochloride Market Segmentation
By Dosage Form
- Capsule
- Tablet
By End-User
- Pharmacy
- Chemical Industry
- Others
Key Market players
Eli Lilly, Teva Pharmaceutical Industries, Dr. Reddy’s Laboratories, Cipla, Sun Pharmaceutical Industries, Aurobindo Pharma, Zydus Lifesciences, Lupin, Viatris (Mylan), Apotex, Glenmark Pharmaceuticals, Hetero, Torrent Pharmaceuticals, Alkem Laboratories, Amneal PharmaceuticalsRaloxifene Hydrochloride Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Raloxifene Hydrochloride Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Raloxifene Hydrochloride market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Raloxifene Hydrochloride market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Raloxifene Hydrochloride market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Raloxifene Hydrochloride market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Raloxifene Hydrochloride market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Raloxifene Hydrochloride value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Raloxifene Hydrochloride industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Raloxifene Hydrochloride Market Report
- Global Raloxifene Hydrochloride market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Raloxifene Hydrochloride trade, costs, and supply chains
- Raloxifene Hydrochloride market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Raloxifene Hydrochloride market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Raloxifene Hydrochloride market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Raloxifene Hydrochloride supply chain analysis
- Raloxifene Hydrochloride trade analysis, Raloxifene Hydrochloride market price analysis, and Raloxifene Hydrochloride supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Raloxifene Hydrochloride market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Eli Lilly
- Teva Pharmaceutical Industries
- Dr. Reddy’s Laboratories
- Cipla
- Sun Pharmaceutical Industries
- Aurobindo Pharma
- Zydus Lifesciences
- Lupin
- Viatris (Mylan)
- Apotex
- Glenmark Pharmaceuticals
- Hetero
- Torrent Pharmaceuticals
- Alkem Laboratories
- Amneal Pharmaceuticals
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 1.79 Billion |
| Forecasted Market Value ( USD | $ 4.19 Billion |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |