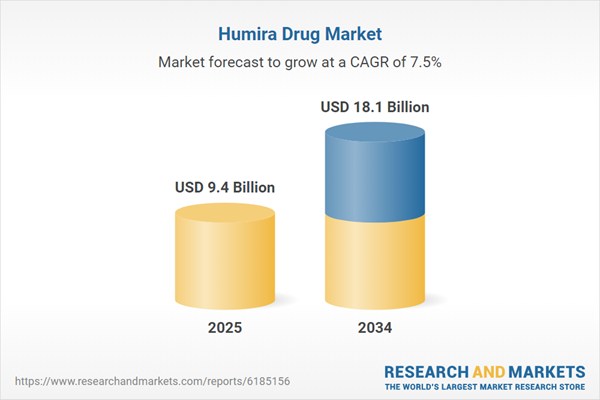
The Humira drug market has long been a dominant force in the biologics and autoimmune disease treatment space. Humira (adalimumab), developed by AbbVie, has been widely prescribed for conditions such as rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, and ankylosing spondylitis. Its mechanism as a TNF-alpha inhibitor has proven highly effective in reducing inflammation and improving patient outcomes. As one of the world’s top-selling drugs for over a decade, Humira has built a substantial footprint in both developed and emerging markets. However, with its patent exclusivity expiring in several regions, biosimilar competition is rapidly reshaping the market. While Humira remains a trusted treatment option, healthcare systems, payers, and physicians are increasingly shifting focus toward cost-effective alternatives. The evolving dynamics have led to significant pricing adjustments, volume-based strategies, and innovation in drug delivery formats, including autoinjectors and prefilled syringes to retain patient loyalty and market share.
The Humira drug market experienced notable transformations, primarily driven by the accelerated entry and adoption of biosimilars across key global markets. In the United States, where Humira’s exclusivity had previously shielded it from competition, multiple biosimilars gained FDA approval and entered the market, prompting pricing shifts and renegotiations with payers. Pharmaceutical companies with approved biosimilars focused heavily on building physician trust, offering competitive reimbursement programs, and marketing comparable efficacy and safety profiles. AbbVie responded by implementing aggressive discounting strategies, introducing its own authorized biosimilar, and expanding partnerships to maintain formulary inclusion. Meanwhile, in Europe and other regions where biosimilars were already established, market penetration deepened further, with national healthcare systems favoring low-cost options to reduce expenditures. Despite declining sales in mature markets, Humira continued to perform well in regions with delayed biosimilar entry and among patients loyal to the original brand due to familiarity and clinical history.
The Humira drug market is expected to undergo continued transition, with biosimilars claiming an even larger share of prescriptions. The long-term outlook points toward a maturing biosimilar landscape, where pricing, accessibility, and value-added services will be key differentiators. AbbVie and other players are likely to shift investment toward next-generation therapies, including more targeted biologics and oral immunomodulators, to offset revenue losses. Patient assistance programs, physician education initiatives, and digital engagement tools will be pivotal in navigating the competitive environment. Emerging markets will offer growth potential as biosimilars gain regulatory approvals and infrastructure for biologics improves. Moreover, innovations in drug delivery, such as longer-acting formulations and improved self-administration devices, will support patient adherence and market sustainability. Regulatory support and harmonization of biosimilar guidelines will further shape global adoption patterns, ensuring a more accessible and competitive therapeutic landscape for autoimmune diseases.
Key Insights: Humira Drug Market
- Rapid expansion of biosimilar launches in major markets is redefining the pricing and competitive structure of the Humira market, prompting more affordable options for healthcare systems.
- Adoption of subcutaneous delivery systems and autoinjectors is growing, improving patient convenience and adherence, particularly among those with chronic autoimmune conditions.
- Pharmaceutical companies are focusing on real-world evidence studies to support biosimilar safety and efficacy, enhancing prescriber confidence and accelerating market penetration.
- Increased reliance on digital tools for patient support, including mobile apps for injection reminders and virtual consultations, is modernizing care delivery models.
- Shift toward personalized immunology treatments is gradually emerging, influencing the development of more targeted biologic drugs beyond Humira’s broad application.
- Growing global prevalence of autoimmune diseases such as rheumatoid arthritis and Crohn’s disease is sustaining high demand for effective biologic therapies like Humira and its biosimilars.
- Regulatory support for biosimilar approvals is accelerating competition and lowering treatment costs, expanding patient access in both developed and emerging economies.
- Increased physician and patient awareness of biosimilars’ clinical comparability to originator drugs is fostering broader market acceptance and prescribing confidence.
- Expanding healthcare infrastructure and biologics reimbursement programs in developing regions are opening new markets for both Humira and competing products.
- Brand loyalty and clinical inertia remain significant barriers, as some patients and providers are hesitant to switch from the originator drug to biosimilars despite cost benefits and comparable outcomes.
Humira Drug Market Segmentation
By Type
- Humira Syringe
- Humira Pen
By Application
- Ankylosing Spondylitis
- Rheumatoid Arthritis
- Crohn's Disease
- Other Applications
By End-Users
- Hospitals
- Specialty Clinics
- Other End-Users
Key Companies Analysed
- Pfizer Inc.
- Merck & Co. Inc.
- AbbVie Inc.
- Novartis AG
- Fresenius Kabi AG
- Amgen Inc.
- Boehringer Ingelheim International GmbH
- Viatris Inc.
- Sandoz International GmbH
- Hikma Pharmaceuticals PLC
- Celltrion Healthcare Co. Ltd.
- Cadila Pharmaceuticals Ltd.
- Torrent Pharmaceuticals Ltd.
- Mochida Pharmaceutical Co. Ltd.
- Innovent Biologics Inc.
- Biocad Biopharmaceutical Company
- Shanghai Henlius Biotech Inc.
- Glenmark Pharmaceuticals Ltd.
- Samsung Bioepis Co Ltd.
- Hetero Healthcare Ltd.
- Coherus BioSciences Inc.
- CinnaGen Co.
- Alvotech Holdings S.A
- Reliance Life Sciences Private Limited
- Momenta Pharmaceuticals Inc.
- Bio-Thera Solutions Ltd.
- Fujifilm Kyowa Kirin Biologics Co. Ltd.
- BioXpress Therapeutics SA
- Alteogen Inc.
- Prestige BioPharma Pte. Ltd.
Humira Drug Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Humira Drug Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Humira Drug market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Humira Drug market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Humira Drug market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Humira Drug market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Humira Drug market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Humira Drug value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Humira Drug industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Humira Drug Market Report
- Global Humira Drug market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Humira Drug trade, costs, and supply chains
- Humira Drug market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Humira Drug market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Humira Drug market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Humira Drug supply chain analysis
- Humira Drug trade analysis, Humira Drug market price analysis, and Humira Drug supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Humira Drug market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Merck & Co. Inc.
- AbbVie Inc.
- Novartis AG
- Fresenius Kabi AG
- Amgen Inc.
- Boehringer Ingelheim International GmbH
- Viatris Inc.
- Sandoz International GmbH
- Hikma Pharmaceuticals PLC
- Celltrion Healthcare Co. Ltd.
- Cadila Pharmaceuticals Ltd.
- Torrent Pharmaceuticals Ltd.
- Mochida Pharmaceutical Co. Ltd.
- Innovent Biologics Inc.
- Biocad Biopharmaceutical Company
- Shanghai Henlius Biotech Inc.
- Glenmark Pharmaceuticals Ltd.
- Samsung Bioepis Co Ltd.
- Hetero Healthcare Ltd.
- Coherus BioSciences Inc.
- CinnaGen Co.
- Alvotech Holdings S.A
- Reliance Life Sciences Private Limited
- Momenta Pharmaceuticals Inc.
- Bio-Thera Solutions Ltd.
- Fujifilm Kyowa Kirin Biologics Co. Ltd.
- BioXpress Therapeutics SA
- Alteogen Inc.
- Prestige BioPharma Pte. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 9.4 Billion |
| Forecasted Market Value ( USD | $ 18.1 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |