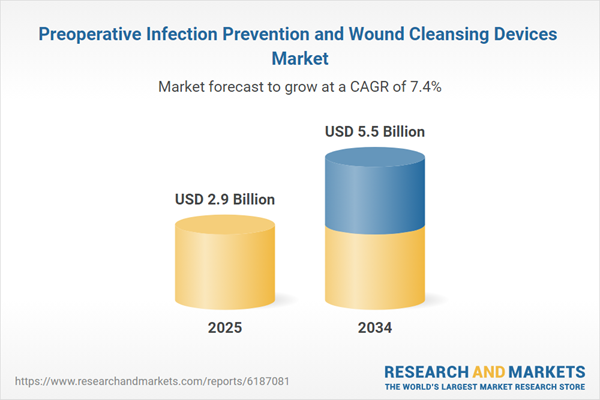
The preoperative infection prevention and wound cleansing devices market plays a crucial role in ensuring the safety and health of patients undergoing surgical procedures. Surgical site infections (SSIs) are one of the most common complications in healthcare settings, making infection prevention critical. Devices designed for preoperative infection prevention, such as antiseptic solutions, wipes, and surgical scrubs, help to significantly reduce the risk of these infections. Wound cleansing devices, including irrigators, antiseptic sprays, and dressings, are used to clean the surgical site before and after procedures, minimizing the likelihood of infection. The global market is expanding due to the increasing number of surgeries performed worldwide, the rising prevalence of chronic diseases requiring surgery, and the growing focus on patient safety. Moreover, advancements in medical technologies and the growing awareness of the importance of infection control in healthcare settings are contributing to the demand for innovative and effective infection prevention and wound cleansing devices. The market is further fueled by the ongoing emphasis on improving healthcare infrastructure and the increasing implementation of stringent sterilization and infection control protocols across hospitals and clinics.
The preoperative infection prevention and wound cleansing devices market saw significant innovations, with the introduction of advanced wound cleansing systems and infection prevention devices designed to improve patient outcomes. Manufacturers focused on developing products that provide higher efficacy in preventing infections while also being gentle on patients’ skin. For instance, the development of non-alcoholic antiseptic solutions for preoperative skin cleansing and antimicrobial wound dressings that promote faster healing gained attention. Additionally, the adoption of single-use devices, which help to eliminate the risk of cross-contamination, became more widespread. These developments were particularly relevant due to the growing concerns over hospital-acquired infections (HAIs) and the implementation of stricter infection control regulations in healthcare settings. The increasing awareness about the importance of preoperative infection prevention, along with the rise in outpatient surgeries and minimally invasive procedures, contributed to the growth of this market. However, challenges such as the high costs associated with advanced infection prevention devices and the need for ongoing education and training for healthcare professionals on proper infection prevention techniques remained a challenge in the industry.
The preoperative infection prevention and wound cleansing devices market is expected to continue growing, with ongoing technological advancements and an increasing global focus on patient safety. The market will likely benefit from the development of more personalized infection prevention solutions, tailored to individual patient needs based on factors like skin type, wound severity, and specific surgical procedures. Moreover, the growing adoption of robotic-assisted surgeries and the increasing number of minimally invasive procedures will drive demand for efficient and effective infection prevention devices that can be used in these settings. As healthcare systems continue to prioritize patient outcomes and quality of care, the market will witness an increased emphasis on the integration of infection prevention technologies with electronic health records (EHR) and other healthcare IT systems to ensure seamless infection management. Furthermore, the rise in chronic disease prevalence and the aging global population will lead to more surgeries, further driving the demand for advanced preoperative infection prevention and wound cleansing devices. However, price sensitivity, particularly in emerging markets, and regulatory challenges related to device approvals may act as barriers to market growth.
Key Insights: Preoperative Infection Prevention and Wound Cleansing Devices Market
- Personalized Infection Prevention Solutions: Increasing focus on developing tailored preoperative infection prevention solutions based on patient-specific factors such as skin type and surgical procedure.
- Advancements in Wound Cleansing Technologies: Continued development of more efficient and less invasive wound cleansing technologies aimed at improving patient comfort and accelerating healing.
- Rise in Minimally Invasive Surgeries: The growth of minimally invasive procedures is driving the demand for advanced infection prevention and wound cleansing devices that can be used effectively in these settings.
- Integration of Infection Prevention Devices with EHR: The integration of infection prevention devices with electronic health records (EHR) systems for improved monitoring and management of patient infections.
- Increased Focus on Single-Use Devices: The adoption of single-use infection prevention and wound cleansing devices to eliminate the risk of cross-contamination and enhance patient safety.
- Rising Surgical Procedures: The growing number of surgical procedures globally, particularly in aging populations and those with chronic conditions, is driving the need for effective infection prevention and wound care solutions.
- Increasing Focus on Patient Safety: Healthcare systems are increasingly prioritizing patient safety, leading to a surge in demand for infection prevention and wound cleansing devices.
- Technological Advancements: Continuous innovations in infection prevention technologies, such as non-alcoholic antiseptic solutions and antimicrobial dressings, are driving market growth.
- Regulatory Support for Infection Control: Governments and regulatory bodies are implementing stricter infection control guidelines, promoting the adoption of advanced infection prevention devices in healthcare facilities.
- High Cost of Advanced Devices: The high price of advanced infection prevention and wound cleansing devices remains a barrier to widespread adoption, especially in cost-sensitive markets.
Preoperative Infection Prevention and Wound Cleansing Devices Market Segmentation
By Surgery Type
- Cataract Surgery
- Cesarean Surgery
- Gastric Bypass
- Appendectomy
- Colectomy and Colostomy
- Esophagectomy
- Biopsy
- Cholecystectomy
- Mastectomy
- Cosmetic Surgery
By Product Type
- Preoperative Infection Prevention Devices
- Preoperative Wound Cleansing Devices
By Application
- Preoperative Hair Removal
- Preoperative Skin Preparation
- Intraoperative Wound Irrigation Solution
- Other Applications
Key Companies Analysed
- Cardinal Health Inc.
- Johnson and Johnson Private Limited
- 3M Company
- Medtronic Plc.
- Medline Industries Inc.
- Becton Dickinson and Co.
- Stryker Corporation
- Baxter International Inc.
- Zimmer Biomet Holdings Inc.
- Smith and Nephew Plc.
- Steris Plc.
- BioMerieux SA
- Coloplast AS
- Teleflex Incorporated
- Paul Hartmann AG
- Getinge AB
- Molnlycke Health Care AB
- ConvaTec Group plc.
- Integra LifeSciences Corporation
- Ansell Limited
- ArjoHuntleigh
- Aesculap Inc.
- Halyard Health Inc.
- Hollister Inc.
- DJO Global Inc.
- Welmed Inc.
- Sterimed SAS
- Dynarex Corporation
- Bactiguard AB
- Careon Healthcare Solutions Pvt Ltd.
Preoperative Infection Prevention and Wound Cleansing Devices Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Preoperative Infection Prevention and Wound Cleansing Devices Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Preoperative Infection Prevention and Wound Cleansing Devices market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Preoperative Infection Prevention and Wound Cleansing Devices market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Preoperative Infection Prevention and Wound Cleansing Devices market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Preoperative Infection Prevention and Wound Cleansing Devices market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Preoperative Infection Prevention and Wound Cleansing Devices market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Preoperative Infection Prevention and Wound Cleansing Devices value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Preoperative Infection Prevention and Wound Cleansing Devices industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Preoperative Infection Prevention and Wound Cleansing Devices Market Report
- Global Preoperative Infection Prevention and Wound Cleansing Devices market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Preoperative Infection Prevention and Wound Cleansing Devices trade, costs, and supply chains
- Preoperative Infection Prevention and Wound Cleansing Devices market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Preoperative Infection Prevention and Wound Cleansing Devices market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Preoperative Infection Prevention and Wound Cleansing Devices market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Preoperative Infection Prevention and Wound Cleansing Devices supply chain analysis
- Preoperative Infection Prevention and Wound Cleansing Devices trade analysis, Preoperative Infection Prevention and Wound Cleansing Devices market price analysis, and Preoperative Infection Prevention and Wound Cleansing Devices supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Preoperative Infection Prevention and Wound Cleansing Devices market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Cardinal Health Inc.
- Johnson and Johnson Private Limited
- 3M Company
- Medtronic PLC
- Medline Industries Inc.
- Becton Dickinson and Co.
- Stryker Corporation
- Baxter International Inc.
- Zimmer Biomet Holdings Inc.
- Smith and Nephew PLC
- Steris PLC
- BioMerieux SA
- Coloplast AS
- Teleflex Incorporated
- Paul Hartmann AG
- Getinge AB
- Molnlycke Health Care AB
- ConvaTec Group PLC
- Integra LifeSciences Corporation
- Ansell Limited
- ArjoHuntleigh
- Aesculap Inc.
- Halyard Health Inc.
- Hollister Inc.
- DJO Global Inc.
- Welmed Inc.
- Sterimed SAS
- Dynarex Corporation
- Bactiguard AB
- Careon Healthcare Solutions Pvt Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 2.9 Billion |
| Forecasted Market Value ( USD | $ 5.5 Billion |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |