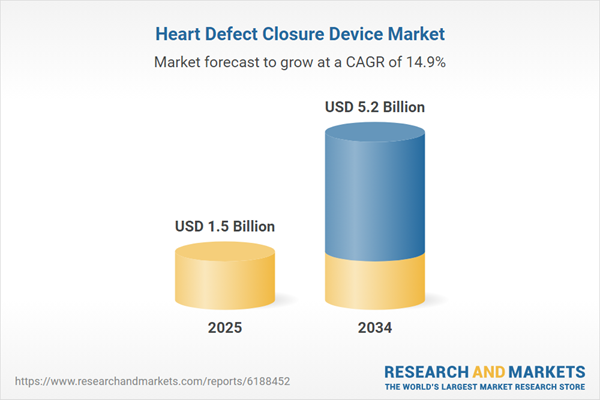
The Heart Defect Closure Device Market is witnessing growing demand due to the rising prevalence of congenital heart defects (CHDs) and structural heart conditions globally. These devices, including septal occluders and transcatheter closure systems, are designed to correct abnormalities in the heart wall, offering minimally invasive alternatives to open-heart surgery. Technological advancements have significantly enhanced device precision, safety, and patient outcomes, making them a preferred treatment option. The market benefits from an aging population, increased awareness of early cardiovascular intervention, and improved diagnostic capabilities. As healthcare systems across the globe adopt more advanced cardiac care solutions, the heart defect closure device market is poised for steady growth, supported by strong investments from medical device companies and increasing procedural volumes in both pediatric and adult populations.
The Heart Defect Closure Device Market experienced noticeable innovation and expansion. Major players introduced next-generation closure devices with enhanced biocompatibility and lower risk of post-procedural complications, addressing long-standing clinical concerns. The year also saw an increase in regulatory approvals, allowing for wider product availability across emerging economies. Demand surged in outpatient settings, driven by advancements in catheter-based interventions and shorter recovery times. Clinical trials focused on evaluating device effectiveness in complex or residual defects, expanding the eligible patient base. Furthermore, partnerships between device manufacturers and academic institutions led to development of customizable solutions tailored for individual anatomical variations. Reimbursement improvements in key regions, particularly the U.S. and parts of Europe, also bolstered patient access and provider confidence in adopting the latest technologies.
The Heart Defect Closure Device Market is expected to gain momentum through broader integration of 3D imaging and AI-based planning tools that enhance procedural accuracy and personalization. Market players are anticipated to invest in bioresorbable materials, offering temporary support with long-term safety benefits. Geographic expansion into underpenetrated regions in Asia-Pacific and Latin America will open new growth avenues as awareness and healthcare infrastructure improve. Continued miniaturization of devices will facilitate their use in neonates and infants, broadening application across all age groups. Strategic mergers and acquisitions are likely to shape the competitive landscape, enabling faster innovation cycles and access to complementary technologies. As heart defect management becomes more proactive and less invasive, the market is set to transform from niche to mainstream in structural heart care.
Key Insights: Heart Defect Closure Device Market
- Development of patient-specific heart defect closure devices using 3D printing technology is gaining traction for better anatomical fit and reduced complication rates.
- Increasing use of AI-driven imaging software to assist interventional cardiologists in mapping and guiding closure procedures with higher precision and reduced operating times.
- Emergence of biodegradable closure devices that dissolve over time, reducing the need for long-term monitoring or secondary interventions.
- Rise in hybrid cardiac procedures combining surgical and catheter-based techniques, expanding treatment options for complex heart defects.
- Growth in outpatient and ambulatory cardiac centers offering same-day heart defect closure procedures with minimally invasive approaches.
- Rising global incidence of congenital and acquired heart defects is driving demand for safe and effective closure solutions across pediatric and adult patients.
- Advancements in interventional cardiology technologies and techniques are enabling less invasive, more efficient treatment options with quicker recovery.
- Favorable reimbursement frameworks and insurance coverage in developed markets are encouraging both hospitals and patients to opt for advanced closure devices.
- Growing awareness about early detection and timely intervention of heart defects, supported by improved diagnostic imaging tools and public health initiatives.
- High costs of advanced closure devices and procedures, combined with limited availability of specialized cardiac care in low-income regions, remain key challenges to widespread adoption.
Heart Defect Closure Device Market Segmentation
By Type
- Atrial Septal Defect (ASD) Closure Device
- Left Atrial Appendage (LAA) Closure Devices
- Patent Foramen Ovale (PFO) Closure Devices
- Patent Ductus Arteriosus (PDA) Closure Devices
- Ventricular Septal Defect (VSD) Closure Devices
By Material
- Nitinol-Based Devices
- Stainless Steel Devices
- Other Materials
By Mode Of Delivery
- Transcatheter Delivery
- Surgical Delivery
- Other Modes
By End-User
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Other End-Users
Key Companies Analysed
- Abbott Laboratories
- Medtronic
- Koninklijke Philips N.V.
- Boston Scientific Corporation
- Terumo Corporation
- Edwards Lifesciences
- W L Gore and Associates
- B. Braun
- BIOTRONIK SE & Co. KG
- Lepu Medical Technology
- Meril Life Sciences Pvt. Ltd.
- MicroPort Scientific Corporation
- AtriCure Inc.
- LifeTech Scientific Corporation
- Baylis Medical Company Inc.
- Biosense Webster Inc.
- Sahajanand Medical Technologies Limited
- Occlutech
- Coherex Medical Inc.
- Cardia Inc.
- Acrostak Corporation
- Vivasure Medical
- Transmural Systems Inc.
- Cormatrix Cardiovascular Inc.
- and St. Jude Medical LLC.
Heart Defect Closure Device Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modeling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behavior are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Heart Defect Closure Device Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Heart Defect Closure Device market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Heart Defect Closure Device market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Heart Defect Closure Device market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Heart Defect Closure Device market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Heart Defect Closure Device market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Heart Defect Closure Device value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Heart Defect Closure Device industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Heart Defect Closure Device Market Report
- Global Heart Defect Closure Device market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Heart Defect Closure Device trade, costs, and supply chains
- Heart Defect Closure Device market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Heart Defect Closure Device market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Heart Defect Closure Device market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Heart Defect Closure Device supply chain analysis
- Heart Defect Closure Device trade analysis, Heart Defect Closure Device market price analysis, and Heart Defect Closure Device supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Heart Defect Closure Device market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Medtronic
- Koninklijke Philips N.V.
- Boston Scientific Corporation
- Terumo Corporation
- Edwards Lifesciences
- W L Gore and Associates
- B. Braun
- BIOTRONIK SE & Co. KG
- Lepu Medical Technology
- Meril Life Sciences Pvt. Ltd.
- MicroPort Scientific Corporation
- AtriCure Inc.
- LifeTech Scientific Corporation
- Baylis Medical Company Inc.
- Biosense Webster Inc.
- Sahajanand Medical Technologies Limited
- Occlutech
- Coherex Medical Inc.
- Cardia Inc.
- Acrostak Corporation
- Vivasure Medical
- Transmural Systems Inc.
- Cormatrix Cardiovascular Inc.
- and St. Jude Medical LLC.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | October 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 1.5 Billion |
| Forecasted Market Value ( USD | $ 5.2 Billion |
| Compound Annual Growth Rate | 14.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |