In addition, increasing regulatory compliance and quality assurance standards, as well as the expansion of small and virtual pharma companies, are propelling market growth. The growing prevalence of poorly soluble APIs and advanced targeted drug delivery systems has driven significant demand for specialized expertise in oral solid dosage (OSD) formulations. U.S. contract development and manufacturing organizations (CDMOs) that can manage complex formulations such as controlled-release tablets, fixed-dose combinations, and nanotechnology in drug delivery are attracting outsourcing contracts from global pharmaceutical companies. For instance, in August 2025, Piramal Pharma Solutions inaugurated a dedicated OSD suite in Pennsylvania, investing millions to support fixed-dose combination production and enhance operational efficiency through advanced granulation, compression, tableting, and coating capabilities. Thus, by offering these sophisticated services, CDMOs help clients overcome formulation challenges, improve therapeutic efficacy, and ensure product stability, differentiating themselves, optimizing facility capacity, and building long-term partnerships with innovators seeking high-value solutions.
Further, pharmaceutical companies face pressure to reduce time-to-market, operational costs, and ensure high-quality standards for their products. Outsourcing oral solid dosage (OSD) manufacturing to U.S.-based CDMOs allows companies to utilize specialized expertise, optimize resources, and streamline supply chains, enabling faster and more efficient product development. For instance, in September 2024, Thermo Fisher Scientific invested USD 22 million to expand OSD development and manufacturing at Cincinnati, Ohio, and Bend, Oregon, enhancing early-stage R&D, formulation, and testing capabilities within its CDMO and CRO network. Furthermore, by collaborating with CDMOs, companies can avoid the costs of building and maintaining in-house facilities and regulatory capabilities. Therefore, CDMOs that provide comprehensive services, from formulation development and scale-up to commercial manufacturing and packaging, offer a strategic advantage for accelerating product launches.
Moreover, stringent FDA regulations and evolving global quality standards are major drivers for outsourcing oral solid dosage (OSD) manufacturing to U.S. CDMOs. Maintaining in-house regulatory expertise and infrastructure is expensive and complex, prompting pharmaceutical companies to rely on partners with proven compliance track records. In addition, CDMOs adhering to cGMP, FDA inspection readiness, and international quality requirements provide risk mitigation, audit preparedness, and consistent product safety. This regulatory excellence builds client trust and positions CDMOs as preferred outsourcing partners. As a result, companies gain recurring contracts, long-term collaborations, and enhanced market credibility.
U.S. Oral Solid Dosage CDMO Market Report Segmentation
This report forecasts revenue growth at the country level and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2033. The report has segmented the U.S. oral solid dosage CDMO market report based on product, mechanism, technology, drug potency, and end use:Product Outlook (Revenue, USD Million, 2021-2033)
- Tablets
- Capsules
- Powders
- Granules
- Others
Mechanism Outlook (Revenue, USD Million, 2021-2033)
- Immediate Release
- Delayed Release
- Controlled Release
Technology Outlook (Revenue, USD Million, 2021-2033)
- Granulation Technologies
- Compression Technologies
- Encapsulation Technologies
- Coating Technologies
- Continuous Manufacturing Technologies
- Others
Drug Potency Outlook (Revenue, USD Million, 2021-2033)
- High Potent Drugs
- Moderate Potent Drugs
- Low Potent Drugs
End Use Outlook (Revenue, USD Million, 2021-2033)
- Large Size Companies
- Medium & Small Size Companies
- Others
Why You Should Buy This Report
- Comprehensive Market Analysis: Gain detailed insights into the market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listings for you to stay ahead of the curve
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The companies profiled in this U.S. Oral Solid Dosage CDMO market report include:- Lonza
- Thermo Fisher Scientific Inc.
- Cambrex Corporation
- Catalent Inc.
- Siegfried Holding AG
- Recipharm AB
- CordenPharma International
- Boehringer Ingelheim
- Piramal Pharma Solutions
- Aenova Group
- Almac Group
- Jubilant Pharmova Limited
- AbbVie Contract Manufacturing
- Quotient Sciences
- SPI Pharma
- DPT Laboratories Ltd.
- Alcami Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 162 |
| Published | December 2025 |
| Forecast Period | 2024 - 2033 |
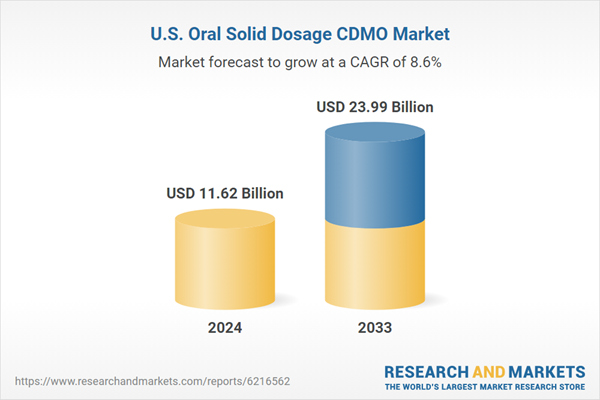
| Estimated Market Value ( USD | $ 11.62 Billion |
| Forecasted Market Value ( USD | $ 23.99 Billion |
| Compound Annual Growth Rate | 8.6% |
| Regions Covered | United States |
| No. of Companies Mentioned | 18 |