POCT solutions enable immediate diagnostic results outside conventional laboratory environments, enhancing clinical efficiency and patient outcomes. These technologies play a pivotal role in managing chronic illnesses and infectious diseases, particularly within Japan’s aging society. Government-backed healthcare initiatives, coupled with ongoing innovation in diagnostic technologies, are accelerating adoption across homecare and clinic-based settings. As Japan continues to emphasize efficiency, accessibility, and precision in healthcare delivery, POCT is becoming an integral pillar of its diagnostic infrastructure.
Market Drivers and Restraints
Rising Demand Due to Aging Demographic Profile
Japan’s population aged 65 years and above surpassed 36 million in 2024, accounting for over 29% of the total population. This demographic shift has intensified pressure on healthcare systems to manage long-term conditions such as diabetes, cardiovascular disorders, and respiratory diseases. POCT addresses these challenges by enabling immediate testing, minimizing hospital visits, and supporting Japan’s expanding homecare model.POCT devices specifically designed for elderly users - featuring simplified interfaces, voice prompts, and portable formats - are increasingly deployed in nursing homes and community clinics. In 2024 alone, more than 1 million elderly individuals required routine health monitoring, significantly boosting demand for frequent and localized testing. Government funding initiatives continue to accelerate POCT integration into eldercare services, positioning the aging population as a primary growth driver.
Technology Integration Accelerates Market Transformation
Japan’s advanced technological ecosystem is reshaping the POCT landscape through widespread adoption of mobile-connected and Bluetooth-enabled diagnostic devices. In 2024, more than 800,000 mobile POCT units were sold nationwide, reflecting rising acceptance of connected healthcare solutions. These devices enable real-time transmission of test results to physicians, particularly across metropolitan regions such as Tokyo, Osaka, and Yokohama.Domestic innovators are leading this transformation, with over 150,000 mobile POCT units activated each month in clinical environments. These technologies improve chronic disease management and strengthen patient engagement. Companies collaborating with Japan’s electronics and telecommunications sectors to develop secure, user-friendly diagnostic platforms are well positioned to gain competitive advantage.
Challenges: Trust and Accuracy Limitations
Despite strong demand, concerns related to test accuracy and result consistency remain a critical challenge. In 2024, over 120,000 POCT-related cases - primarily involving glucose and lipid testing - reported discrepancies linked to user handling errors or calibration issues. In Japan’s healthcare environment, where precision is paramount, such incidents negatively affect clinician and patient confidence.Regulatory authorities issued warnings for more than 50,000 defective POCT devices in 2024, including a major recall of hemoglobin test kits impacting 80,000 users. These events have reinforced the need for enhanced quality control, user training, and validation protocols. Adoption of AI-based error detection and closer collaboration with the Japanese Ministry of Health are becoming essential to address these limitations.
Market Trends and Opportunities
Expansion of Mobile Testing Ecosystems
The rapid growth of mobile device usage is transforming diagnostic delivery across Japan. App-connected and Bluetooth-enabled POCT tools enable real-time data sharing and seamless integration with electronic health records (EHRs). Cholesterol and glucose self-monitoring kits are witnessing strong adoption among urban, tech-savvy populations.Partnerships between diagnostic manufacturers and mobile service providers are emerging, enabling bundled offerings that combine POCT kits with connectivity solutions. Healthcare facilities in cities such as Nagoya and Fukuoka are reporting increased uptake of mobile-compatible diagnostics, highlighting scalable growth opportunities through telecom-health collaborations.
Prescription-Based Testing Anchors Clinical Trust
Physician-directed testing continues to dominate the POCT landscape in Japan. In 2024, more than 3 million POCT procedures were ordered annually through prescriptions, supported by Japan’s universal healthcare coverage and strong regulatory oversight. This model ensures reliability and seamless integration into clinical workflows.Large hospitals alone conduct over 200,000 cardiac marker POCTs each month, underscoring POCT’s importance in both emergency and chronic care settings. Companies investing in physician-facing platforms and clinically validated diagnostics are best positioned to sustain long-term adoption.
Segmental Intelligence
Testing Kits & Consumables Maintain Market Dominance
Testing Kits & Consumables represent the largest revenue-generating product category, encompassing glucose strips, rapid antigen kits, lipid panels, and reagents. Unlike diagnostic instruments, which involve one-time capital investment, consumables generate recurring revenue aligned with Japan’s high testing frequency.In 2024, over 500,000 rapid respiratory antigen test kits were distributed during peak infection seasons, while monthly sales of glucose strips remained consistently strong. Their affordability, scalability, and disposability position consumables as the core revenue driver within the POCT market.
Infectious Diseases Emerge as Prime Testing Area
The Infectious Diseases segment remains the leading application area for POCT in Japan due to dense urban populations and recurring seasonal outbreaks. More than 2.5 million infectious disease POCTs were administered in 2024, with influenza and norovirus accounting for a substantial share.The reporting of 1.8 million influenza cases in 2024 underscores the importance of rapid, large-scale testing. Diagnostic providers offering cost-effective, high-sensitivity kits with fast turnaround times are well positioned to secure large institutional contracts.
Immunological PoC Tests Lead by Technology and Application
Immunological PoC Tests dominate Japan’s POCT market due to their accuracy, speed, and versatility across clinical applications. By mid-2024, more than 1.2 million immunological kits were actively used across healthcare settings.Rapid antigen tests for COVID-19, hepatitis, and influenza are widely deployed in hospitals, homecare, and rural clinics. Their ability to deliver results within 15 minutes supports immediate clinical decision-making, particularly in elderly care and emergency settings.
Regulatory Environment and Market Entry Considerations
Japan’s stringent regulatory framework ensures high diagnostic reliability while presenting entry challenges for new market participants. Foreign manufacturers must meet rigorous performance and quality standards. However, partnerships with local distributors and medical institutions can significantly streamline market entry and accelerate trust.Global players such as QIAGEN, F. Hoffmann-La Roche Ltd, and Abbott Laboratories have successfully expanded their presence by localizing products and collaborating with domestic companies including Astellas Pharma Inc. The rollout of Accu-Chek Guide Me in partnership with BlueStar demonstrates how co-development strategies accelerate market adoption.
Competitive Intelligence
The Japan point-of-care testing market is highly competitive, driven by innovation from both domestic and global players.Domestic Leaders:
- Sysmex Corporation
- SEKISUI MEDICAL CO., LTD.
Global Key Players:
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- Sekisui Diagnostics
- BD (Becton, Dickinson and Company)
- FUJIFILM
- Other Prominent Players
Market Segmentation Overview
By Product
- Devices & Instruments
- Testing Kits & Consumables
By Test Type
- Immunological PoC Tests
- Nucleic Acid-Based PoC Tests
- Biomarker-Based PoC Tests
By Indication
- Infectious Diseases (HIV, COVID-19, Others)
- Oncology
- Cardiovascular Diseases
- Metabolic Disorders
- Respiratory Diseases
- Neurological Disorders
- Gastrointestinal Disorders
- Others
By Technology
- Biosensor Technology
- Microfluidic Lab-On-A-Chip Technology
- Molecular Diagnostics
- Immunoassays
- Others
By Sample Type
- Blood
- Urine
- Saliva
- Others
By Mode of Purchase
- Prescription-based Testing
- Over the Counter (OTC) Testing
By End User
- Hospitals & Clinics
- Diagnostic Centers
- Homecare Settings
- Research Laboratories
- Others
By Distribution Channel
- Direct Distribution
- Retail Pharmacies
- Online Pharmacies
- Others
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- Sekisui Diagnostics
- BD (Becton, Dickinson and Company)
- FUJIFILM
- Sysmex Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | July 2025 |
| Forecast Period | 2024 - 2033 |
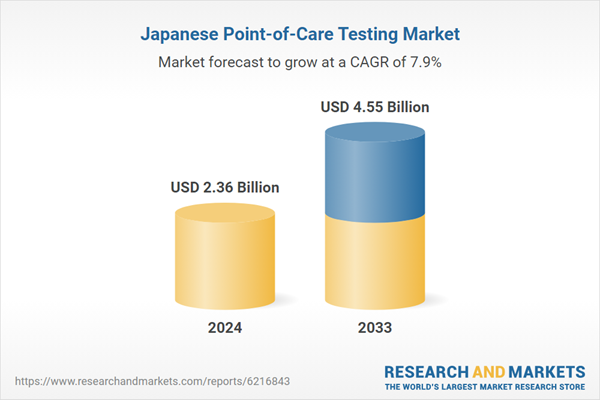
| Estimated Market Value ( USD | $ 2.36 Billion |
| Forecasted Market Value ( USD | $ 4.55 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Japan |