Speak directly to the analyst to clarify any post sales queries you may have.
Understanding the Fundamental Biology and Critical Therapeutic Potential of Hedgehog Pathway Inhibitors in Oncology and Rare Disease Applications
The Hedgehog signaling pathway plays a pivotal role in embryonic development tissue patterning and stem cell maintenance across vertebrate species. Aberrant activation of this pathway has been implicated in a spectrum of cancers including Acute Myeloid Leukemia Basal Cell Carcinoma and Medulloblastoma. By modulating the activity of proteins such as Smoothened and Gli transcription factors Hedgehog pathway inhibitors offer a targeted therapeutic approach designed to disrupt oncogenic signaling cascades at their source.
Preclinical studies have illuminated how pathway blockade can induce tumor regression overcome resistance mechanisms and synergize with established chemotherapies. Clinical validation in patients with basal cell carcinoma has demonstrated durable responses leading to regulatory approvals and an expanding pipeline of small molecules antibodies and RNAi modalities. In parallel researchers are investigating novel combination regimens and biomarkers to refine patient selection and maximize therapeutic benefit.
This introduction sets the stage for a deeper examination of market dynamics technological advances regulatory influences and strategic imperatives that will define the trajectory of Hedgehog pathway inhibitors in oncology and rare disease landscapes.
Assessing Revolutionary Advances and Paradigm Shifts Redefining the Hedgehog Pathway Inhibitor Landscape from Bench to Approved Therapeutic Interventions
Recent years have witnessed paradigm shifts that are redefining the landscape of Hedgehog pathway inhibitors from early-stage discovery through late-stage clinical development. Advances in structural biology have elucidated the conformational changes of Smoothened receptor domains enabling structure-guided design of next generation antagonists with enhanced potency and selectivity profiles. These insights have accelerated the translation of novel small molecules and biologic modalities into the clinic.
Simultaneously the convergence of precision oncology and advanced molecular diagnostics has empowered investigators to identify patient subpopulations most likely to derive durable benefit from pathway inhibition. Emerging combination strategies are pairing Hedgehog inhibitors with immune checkpoint blockade kinase inhibitors or epigenetic modulators to overcome adaptive resistance and achieve deeper remissions. The integration of digital health technologies such as remote monitoring and real time biomarker analysis is optimizing trial execution and enriching datasets with longitudinal patient-reported outcomes.
Moreover evolving regulatory frameworks are encouraging accelerated approval pathways based on robust surrogate endpoints and real-world evidence. These transformative shifts underscore the rapid maturation of the field and highlight strategic opportunities for stakeholders across the value chain.
Analyzing the Far-Reaching Effects of United States Tariff Reforms Announced for 2025 on Hedgehog Pathway Inhibitor Supply Chains and Cost Structures
The United States has announced a series of tariff adjustments scheduled to take effect in 2025 that will influence the importation of active pharmaceutical ingredients raw materials and specialized biologics associated with Hedgehog pathway inhibitor development. These measures are poised to alter cost structures for manufacturers reliant on global supply chains extending from Asia to Europe. For organizations sourcing critical reagents overseas the revised duty schedules may necessitate strategic relocation of manufacturing hubs or renegotiation of supplier agreements to preserve margin profiles.
At the same time downstream distributors and contract research organizations may experience upward pressure on service fees that could affect overall R&D budgets. Sponsors are evaluating dual sourcing strategies and applying tariff engineering approaches to reclassify materials under more favorable trade categories. Longer lead times for raw material procurement are anticipated prompting stakeholders to enhance inventory planning and invest in risk mitigation protocols.
In response regulatory affairs teams are closely monitoring tariff announcements to ensure compliance and maintain uninterrupted clinical supply. Early scenario planning, cross-functional collaboration and proactive negotiations with customs authorities will be essential to offset potential revenue impacts and sustain momentum across key therapeutic programs.
Unveiling Key Segmentation Dynamics That Drive Growth and Diversification in the Hedgehog Pathway Inhibitor Market Across Multiple Commercial Criteria
Market segmentation analysis reveals distinct growth drivers and strategic priorities across indication product type route of administration end user and distribution channel. When viewed through the lens of indication the focus on Acute Myeloid Leukemia underscores an unmet need for targeted hematologic therapies while Basal Cell Carcinoma advocates for broader ambulatory use and Medulloblastoma emphasizes pediatric oncology applications. Each of these submarkets demands tailored clinical trial designs and regulatory strategies to address unique safety and efficacy considerations.
From a product type perspective the emergence of antibody-based and RNAi approaches is challenging the hegemony of small molecules by offering higher specificity and novel mechanisms of pathway blockade. Oral formulations continue to dominate due to patient convenience and outpatient applicability yet parenteral delivery channels remain critical for investigational biologics and inpatient administration settings.
Hospitals and research institutes represent primary end user segments driving early adoption through clinical studies and hospital-led formulary decisions while specialty clinics are positioned to facilitate community-based treatment of basal cell carcinoma. Distribution through hospital pharmacies retains its central role for inpatient care, whereas online pharmacies enhance patient access in homecare scenarios and retail outlets support retail prescribing models for chronic indications.
Illuminating Regional Market Behaviors and Strategic Opportunities for Hedgehog Pathway Inhibitors Across Americas Europe Middle East & Africa and Asia-Pacific Clusters
Regional market behaviors for Hedgehog pathway inhibitors are shaped by variations in healthcare infrastructure reimbursement policies and regulatory requirements across global zones. In the Americas, advanced oncology networks and favorable patent frameworks have fostered rapid adoption of novel therapeutics accompanied by growing investment in clinical trial sites. Reimbursement pathways in Europe Middle East & Africa exhibit greater heterogeneity with some markets prioritizing cost-effectiveness assessments and health technology appraisals, while others leverage orphan drug incentives to expedite approvals for rare disease indications.
Emerging economies within the Asia-Pacific region are investing heavily in biotechnology capabilities and forging public-private partnerships to strengthen local production of small molecules and biologics. Regulatory harmonization efforts are streamlining dossier submissions and facilitating market entry, thereby creating new opportunities for global players and regional innovators alike. Moreover, payer models in this region are evolving to accommodate value-based contracting especially for high-cost oncology agents.
These distinct regional trends underscore the importance of tailoring market entry strategies and lifecycle management plans to align with local reimbursement dynamics clinical priorities and patient access initiatives.
Profiling Pioneering Biopharmaceutical Leaders and Innovative Strategic Partnerships Shaping the Hedgehog Pathway Inhibitor Arena
Competitive profiling of leading biopharmaceutical organizations reveals a diverse array of strategic investments shaping the Hedgehog pathway inhibitor landscape. Established players have fortified their portfolios through targeted acquisitions and research collaborations to expand their small molecule and biologic pipelines. Innovative mid-sized and emerging biotech companies are distinguishing themselves by advancing next-generation antagonist platforms and leveraging precision medicine partnerships to access companion diagnostic expertise.
Key alliance models include codevelopment agreements with academic research centers to explore combination regimens and public-private consortia aimed at accelerating pediatric oncology programs. Licensing agreements have enabled companies to out-license early-stage assets in exchange for milestone payments and royalty streams, thus de-risking internal development costs while maximizing global reach through localized commercialization partners.
Additionally, dedicated venture capital and corporate venture arms are deploying capital to underpin novel RNAi and antibody modalities, reflecting a broader commitment to diversify therapeutic mechanisms. Cross-sector collaborations with diagnostic and digital health firms are also on the rise, underscoring the shift toward integrated treatment solutions that combine targeted inhibition with robust patient monitoring and personalized dosing regimens.
Outlining High-Impact Actionable Recommendations Empowering Industry Stakeholders to Navigate Challenges and Seize Opportunities in Hedgehog Pathway Therapeutics
Industry stakeholders should prioritize the integration of precision diagnostics into early clinical development to enhance patient stratification and accelerate regulatory approvals. By leveraging genomic and proteomic biomarkers, sponsors can optimize cohort selection, reduce trial timelines and strengthen the evidence base for accelerated pathways. Concurrently, companies should explore combination regimens pairing Hedgehog inhibitors with immune or epigenetic agents to address resistance mechanisms and extend therapeutic durability.
Supply chain resilience must be bolstered through comprehensive risk assessments and alternative sourcing strategies aimed at mitigating the impact of impending tariff changes. This includes establishing redundant supplier networks for critical reagents and adopting advanced inventory management technologies to maintain uninterrupted clinical and commercial supply. Proactive engagement with policy makers and customs authorities will also be essential to navigate evolving trade landscapes and safeguard cost efficiencies.
Finally, forging strategic alliances with digital health providers can enhance patient engagement and real-time monitoring, delivering richer safety and efficacy datasets that support market access negotiations. By implementing these actionable recommendations, industry leaders can position themselves to capitalize on emerging opportunities while mitigating operational and regulatory risks.
Detailing a Robust Multimodal Research Methodology Employed to Generate Reliable Insights and Validate Findings Within the Hedgehog Pathway Inhibitor Domain
This research initiative employed a comprehensive multimodal methodology to ensure rigor and validity across all analytical components. The process began with secondary research encompassing peer-reviewed journals conference proceedings patent databases and regulatory filings to build foundational knowledge around Hedgehog pathway biology and therapeutic evolution. Proprietary databases were then consulted to identify clinical pipeline assets competitive landscapes and historical approval trajectories.
Primary research comprised structured interviews with oncology clinicians regulatory experts supply chain executives and patient advocacy representatives to capture firsthand insights on clinical practice patterns, reimbursement landscapes and patient access barriers. Responses were triangulated against secondary data to resolve discrepancies and validate emerging hypotheses. Additionally, a series of workshops with cross-functional stakeholders including pharmacology specialists and market access advisers ensured comprehensive coverage of commercial and scientific considerations.
Quantitative analyses were supplemented by scenario planning exercises to model the impact of tariff changes and regional policy shifts on stakeholder decision making. Finally, all findings underwent a three-stage quality review encompassing technical, editorial and client validation to guarantee accuracy transparency and actionable relevance.
Synthesizing Core Observations Strategic Imperatives and Forward-Looking Vision to Conclude the Executive Summary of Hedgehog Pathway Inhibitor Market Analysis
In conclusion the Hedgehog pathway inhibitor arena stands at the nexus of scientific innovation regulatory evolution and shifting market dynamics. Fundamental insights into pathway structure and function have catalyzed the development of targeted therapeutics across acute myeloid leukemia basal cell carcinoma and medulloblastoma, while advancing modalities such as antibodies and RNAi expand the mechanistic toolkit available to developers.
Regulatory reforms and tariff realignments underscore the necessity for agile supply chain strategies and proactive policy engagement. Regional market behaviors highlight the imperative of customized market entry and reimbursement approaches, particularly in emerging markets where harmonization efforts are gaining momentum. Competitive intelligence demonstrates the value of strategic alliances that integrate diagnostic capabilities and digital health solutions to differentiate offerings and enhance patient outcomes.
By synthesizing these observations and strategic imperatives, stakeholders can formulate cohesive plans that balance innovation with operational resilience. The collective insights presented herein illuminate a pathway forward grounded in evidence based decision making and collaborative execution.
Additional Product Information:
- Purchase of this report includes 1 year online access with quarterly updates.
- This report can be updated on request. Please contact our Customer Experience team using the Ask a Question widget on our website.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Hedgehog Pathway Inhibitors Market
Companies Mentioned
The key companies profiled in this Hedgehog Pathway Inhibitors market report include:- Bristol Myers Squibb Company
- Curis Inc.
- Eli Lilly and Company
- Erasca Inc
- Genentech Inc
- Inflection Biosciences Limited
- Kintara Therapeutics Inc
- Medicenna Therapeutics Corp
- Merck & Co Inc
- Novartis AG
- Pfizer Inc
- Puma Biotechnology Inc
- Roche Holding AG
- Sun Pharmaceutical Industries Ltd
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
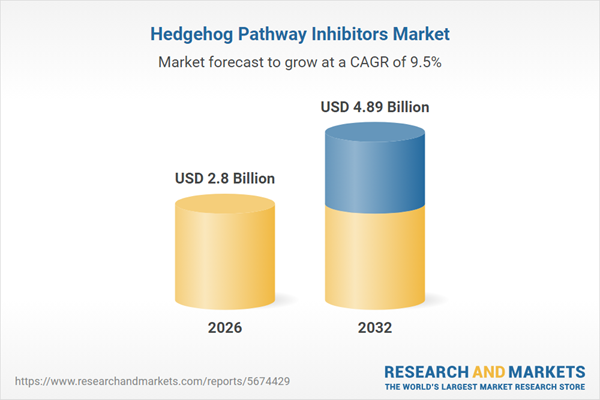
| Estimated Market Value ( USD | $ 2.8 Billion |
| Forecasted Market Value ( USD | $ 4.89 Billion |
| Compound Annual Growth Rate | 9.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |