Recombinant Proteins is the fastest growing segment, North America is the largest regional market
Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
The global viral clearance market is significantly driven by the expanding biopharmaceutical and biologics pipeline, which continually introduces a greater volume and diversity of products necessitating stringent viral safety assurance. As pharmaceutical companies intensify research and development efforts, particularly in advanced therapies, the demand for robust viral clearance solutions escalates across all manufacturing stages, from cell line development to final product formulation. The inherent complexity of novel biologics, including cell and gene therapies, mandates sophisticated viral clearance strategies to meet evolving product specifications and ensure patient safety. According to J.P. Morgan, in December 2024, their '2024 Biopharma Industry Insights: Investment Trends, M&A Activity, and Market Dynamics' report indicated that biologics investment for seed and series A rounds reached $3.5 billion, underscoring the substantial financial commitment fueling this pipeline expansion and, consequently, the demand for viral clearance technologies.Key Market Challenges
A significant challenge impeding the expansion of the global viral clearance market is the substantial investment and specialized technical expertise necessary for developing and validating effective viral clearance strategies, especially for new and varied biopharmaceutical products. This requirement places a considerable financial burden on manufacturers, particularly for smaller and emerging companies, limiting their capacity to adopt or innovate in viral clearance technologies. The complexity associated with ensuring viral safety for novel modalities, such as gene therapies or advanced protein therapeutics, demands extensive research and development resources, along with highly skilled personnel to navigate intricate regulatory pathways and perform rigorous validation studies.Key Market Trends
The global viral clearance market is significantly influenced by the strategic shift towards integrating viral clearance assessments into the early stages of drug development. This proactive approach aims to identify and mitigate potential viral risks much sooner, ultimately streamlining the development timeline and reducing costly delays in later phases. Early consideration of viral clearance strategies also allows for the optimization of purification processes from the outset, ensuring regulatory compliance and product safety as a core component of the development paradigm rather than an afterthought. According to the Food and Drug Administration's Center for Drug Evaluation and Research, in June 2025, their "Analysis of Virus Clearance for Biotechnology Manufacturing Processes from Early to Late Phase Development" article highlighted that the risk for virus contamination in biotechnology products derived from mammalian cell lines must be evaluated from the early stages of development, underscoring this trend's regulatory and scientific importance.Key Market Players Profiled:
- Charles River Laboratories International, Inc.
- Wuxi Biologics Inc
- Eurofins Scientific SE
- Sartorius AG
- Texcell, Inc.
- Biosafety Testing Services, Inc.
- Avance Biosciences Laboratories Inc.
Report Scope:
In this report, the Global Viral Clearance Market has been segmented into the following categories:By Application:
- Recombinant Proteins
- Blood
- Vaccines
- Others
By Method:
- Viral Removal Method
- Viral Inactivation Method
- Viral Detection Method
By End User:
- Pharmaceutical & Biotechnology Companies
- CROs
- Others
By Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Viral Clearance Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The companies profiled in this Viral Clearance market report include:- Charles River Laboratories International, Inc.
- Wuxi Biologics Inc
- Eurofins Scientific SE
- Sartorius AG
- Texcell, Inc.
- Biosafety Testing Services, Inc.
- Avance Biosciences Laboratories Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | November 2025 |
| Forecast Period | 2024 - 2030 |
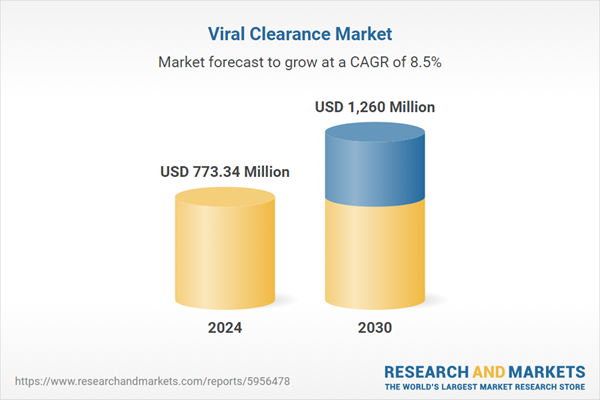
| Estimated Market Value ( USD | $ 773.34 Million |
| Forecasted Market Value ( USD | $ 1260 Million |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 8 |