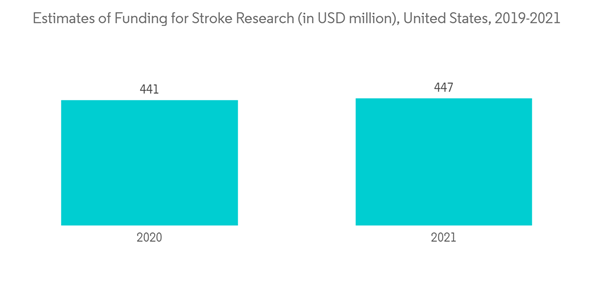The outbreak of COVID-19 affected the neurointerventional devices market in several ways, as it limited the intensive care unit (ICU) beds and ventilation sites owing to the necessity of postponing elective and/or complex cases. Moreover, as per the analysis, there were considerable variations in the management of neuro interventions during and after the pandemic. For instance, as per the report published by Cardiovascular Revascularization Medicine in July 2022, to ascertain the overall effect on vascular intervention trials halted by the COVID-19 pandemic, more research is required in the field of vascular intervention. Hence, the market is expected to rapidly rebound to pre-pandemic levels nonetheless, as a result of the restart of all healthcare facilities, a drop in COVID-19 cases, and follow-up trials on vascular intervention after COVID-19.
The major factors contributing to the growth of the market are the increasing target patient population, ongoing product development and commercialization, and rising research activities in the field of neurovascular therapies.
Neurointerventional devices are used by medical professionals specializing in the domain of the central nervous system (CNS). It is usually done by neuroradiologists, neurosurgeons, and neurologists exploring the endovascular approach to treat the vascular diseases of the central nervous system. The burden of vascular diseases associated with the central nervous system in the target population primarily drives the usage of devices. According to WSO data updated in June 2022, about 12.2 million (12,224,551) people will experience a stroke by the end of the year. In addition, the intracranial aneurysm, also known as a cerebral or brain aneurysm, is a bulging, weakened area in the wall of an artery in the brain. According to data from the National Health Service (NHS), England, updated in April 2022, the prevalence of brain aneurysms could be as high as 1 in 20 people, and brain aneurysms can develop in anyone at any age, but are more common in people over the age of 40. Furthermore, according to updated data from the Brain Aneurysm Foundation in January 2022, an estimated 6.5 million Americans have an unruptured brain aneurysm. About 30,000 people in the United States suffer a brain aneurysm rupture each year. A brain aneurysm ruptures every 18 minutes. The consistently high volume of patients with CNS vascular diseases approaching healthcare facilities is expected to drive the demand for neurointerventional devices.
Similarly, the new device developments and approvals will help the market grow during the study period. For instance, in April 2021, Medtronic received approval from the US FDA for the Pipeline Flex Embolization Device with Shield Technology. The Pipeline Flex Embolization Device diverts blood flow away from a brain aneurysm.
Such factors are altogether contributing to the market’s growth over the analysis period. However, the stringent regulations and dearth of skilled neurosurgeons are the primary limitations to the growth of the studied market.
Neurointerventional Devices Market Trends
Ischemic Stroke Segment is Expected to Hold Major Share of the Market Over the Forecast Period
Ischemic stroke is characterized by the sudden loss of blood circulation to an area of the brain, resulting in a corresponding loss of neurologic function. The major factors that positively impact the segment include the increase in the incidence of ischemic stroke and the developments made by various major players in the market.For instance, as per the WSO's Global Stroke Fact Sheet 2022, there are over 7.6 million new ischemic strokes each year. Globally, over 62% of all incident strokes are ischaemic strokes. This shows a significant rise in cases every year that need proper neurointerventions through neurointerventional devices. Thus, the increase in the incidence of vascular diseases like ischaemic stroke drives the neurointerventional devices market over the forecast period.
Similarly, the recent developments in the devices used for ischemic stroke drove the market growth during the study period. For instance, in June 2022, Rapid Medical enrolled the first patient in the DISTALS Study. The trial is the first-ever FDA investigational device exemption (IDE) trial to examine the safety and effectiveness of mechanical thrombectomy in distal stroke with TIGERTRIEVER 13, the smallest and most adjustable thrombectomy device. With a wide range of applications linked to arterial-based disease correction, there is significant usage of neurointerventional devices. Similarly, in February 2022, MicroPort NeuroTech Limited (MicroPort NeuroTech) received marketing approval from the NMPA for its self-developed Neurohawk Stent Thrombectomy Device (Neurohawk). The Neurohawk is the clot stent retriever launched by MicroPort NeuroTech for acute ischemic stroke treatment.
Thus, given the above-mentioned facts, the studied market is expected to grow over the forecast period.
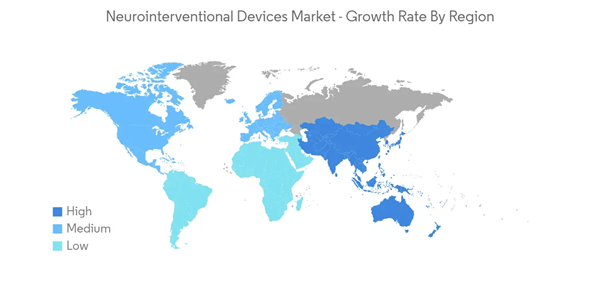
North America is Expected to Hold a Significant Share in the Market Over the Forecast Period
The United States contributes the most to the North American region and is responsible for the dominance of the region across the world. North America, with its advanced healthcare infrastructure across the majority of places and the presence of many medical device companies well-connected with hospitals, primarily boosts the growth of this market.According to the statistics published by the CDC in April 2022, more than 795,000 Americans have strokes. Each year, 610,000 of these instances are discovered to be new. About 87% of all strokes are ischemic strokes, in which blood flow to the brain is blocked. Hence, the increase in stroke cases in the region increases the demand for neurointerventional devices for proper management, which helps in the growth of the market during the forecast period.
Furthermore, new device approvals and launches also help the market grow. For instance, in February 2022, CERENOVUS launched EMBOGUARD, a next-generation balloon guide catheter to be used in endovascular procedures, including those for patients with acute ischemic stroke. The EMBOGUARD balloon guide catheter is designed to optimize the removal of clots by controlling blood flow locally during MT procedures. Thus, all the new device approvals and launches help the market grow in the region.
Thus, all the aforementioned factors are expected to boost the market's growth over the forecast period.
Neurointerventional Devices Market Competitor Analysis
The neurointerventional devices market is moderately competitive and consists of several major players. The companies have identified consistent growth opportunities in the treatment of endovascular-based diseases via neurointerventional devices. Some of the major companies in this segment include Abbott, Johnson & Johnson Services, Inc. (CERENOVUS), Medtronic, Stryker, Penumbra, Inc., MicroPort Scientific Corporation, Terumo Corporation, and W. L. Gore & Associates, Inc.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott
- Johnson & Johnson Services, Inc (CERENOVUS)
- Medtronic
- MicroPort Scientific Corporation
- Penumbra, Inc.
- Stryker
- Terumo Corporation
- W. L. Gore & Associates, Inc.