Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, a significant obstacle to broader market expansion is the difficulty of successfully targeting tissues outside the liver. The natural instability of RNA molecules, combined with the intricacies of manufacturing safe, high-purity delivery systems, presents considerable technical and financial challenges for developers. Consequently, resolving these stability and distribution issues is a vital precondition for securing regulatory approval and achieving the successful commercialization of next-generation RNA therapeutics.
Market Drivers
A major force driving the Global RNA Therapy Clinical Trials Market is the significant influx of venture capital and government funding, which empowers biotechnology companies to maintain capital-intensive research and development efforts. This financial support is crucial for advancing early-stage candidates into later clinical phases, given the substantial costs involved in manufacturing and safety testing. Furthermore, access to capital facilitates pipeline diversification beyond infectious diseases into areas like oncology and rare genetic disorders, thereby lowering systemic risk. As evidence of investor resilience, the Alliance for Regenerative Medicine noted in October 2024 that the broader cell and gene therapy sector, which includes RNA therapeutics, secured $10.9 billion in investment during the first half of the year.In parallel, the proven success of mRNA technology platforms during the pandemic has created a strong basis for market acceleration, transitioning focus from prophylactic vaccines to therapeutic treatments. The demonstrated ability of mRNA to generate protein production in vivo has reduced risks for regulators and investors, prompting the investigation of novel uses such as cancer immunotherapies and protein replacement strategies. This technical maturity is resulting in tangible regulatory achievements that further boost clinical activity. For instance, Moderna reported in May 2024 that the U.S. FDA approved mRESVIA for protecting adults aged 60 and older against respiratory syncytial virus, the first non-COVID mRNA vaccine approval, while the International Society for Cell & Gene Therapy noted that seven distinct cell and gene therapy products received FDA approval throughout 2024, highlighting the regulatory momentum supporting this market.
Market Challenges
The principal hindrance to the Global RNA Therapy Clinical Trials Market is the technical complexity involved in delivering therapies effectively to tissues other than the liver, alongside the inherent instability of RNA molecules. Although existing delivery vehicles such as lipid nanoparticles work well for hepatic targets, adapting them for other organs necessitates complex engineering that often degrades stability or increases toxicity. This constraint compels pharmaceutical developers to invest heavily in intricate manufacturing processes to guarantee high-purity delivery systems, which significantly raises development costs and lengthens timelines. As a result, the elevated risk of clinical failure linked to these stability and distribution issues creates a hesitant investment climate, causing many potential candidates to stall in preclinical stages rather than progressing to human trials.This technical bottleneck creates a measurable drag on the market's rate of expansion. According to the American Society of Gene & Cell Therapy, clinical development activity across the broader gene, cell, and RNA therapy landscape slowed in the third quarter of 2025, with a total of 125 trials initiated across these sectors. This deceleration suggests that despite significant interest in RNA therapeutics, the persistent difficulty in overcoming delivery and stability barriers is impeding the rapid translation of research into active clinical studies, thereby moderating the overall growth trajectory of the market.
Market Trends
The acceleration of personalized mRNA cancer vaccine trials is fundamentally transforming the market by shifting the application of messenger RNA technology from preventing infectious diseases to precision oncology. Developers are utilizing rapid manufacturing capabilities honed during the pandemic to create patient-specific immunotherapies targeting unique tumor neoantigens, aiming to address high recurrence rates in solid tumors. This trend involves a quick progression from early-phase safety studies to large-scale efficacy trials, driven by the necessity to validate the therapeutic potential of mRNA in complex cancer cases. Highlighting this aggressive growth, BioNTech confirmed in its November 2024 financial update an objective to have ten or more potentially registrational trials in its oncology pipeline by the end of 2024, indicating a substantial increase in late-stage development.Simultaneously, the expansion of RNA-editing therapeutic clinical programs marks a significant evolution in the sector, advancing from gene silencing or replacement to the precise correction of genetic mutations at the transcript level. This method employs endogenous cellular machinery, such as ADAR enzymes, to transiently edit RNA sequences without permanently modifying genomic DNA, providing a safer alternative to CRISPR-based editing for chronic genetic disorders. This approach has recently moved from theoretical potential to clinical validation, confirming a new class of therapeutics. For example, Wave Life Sciences reported in October 2024 that its Phase 1b/2a RestorAATion-2 study achieved the first-ever therapeutic RNA editing in humans, with patients treated with WVE-006 showing a mean wild-type M-AAT protein restoration of over 60% of total AAT.
Key Players Profiled in the RNA Therapy Clinical Trials Market
- IQVIA Inc.
- ICON PLC
- Laboratory Corporation of America Holdings
- Charles River Laboratories International, Inc.
- PAREXEL International Corp.
- Syneos Health
- Medpace Holdings, Inc.
- Novotech Inc.
- PPD Inc.
- Veristat, LLC.
Report Scope
In this report, the Global RNA Therapy Clinical Trials Market has been segmented into the following categories:RNA Therapy Clinical Trials Market, by Modality:
- RNA interference
- Antisense therapy
- Messenger RNA
- Oligonucleotide
- non-antisense
- non-RNAi
RNA Therapy Clinical Trials Market, by Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
RNA Therapy Clinical Trials Market, by Therapeutic Areas:
- Rare Diseases
- Anti-infective
- Anticancer
- Neurological
- Alimentary/Metabolic
- Musculoskeletal
- Cardiovascular Respiratory
- Sensory
- Others
RNA Therapy Clinical Trials Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global RNA Therapy Clinical Trials Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this RNA Therapy Clinical Trials market report include:- IQVIA Inc.
- ICON PLC
- Laboratory Corporation of America Holdings
- Charles River Laboratories International, Inc.
- PAREXEL International Corp.
- Syneos Health
- Medpace Holdings, Inc.
- Novotech Inc.
- PPD Inc.
- Veristat, LLC.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
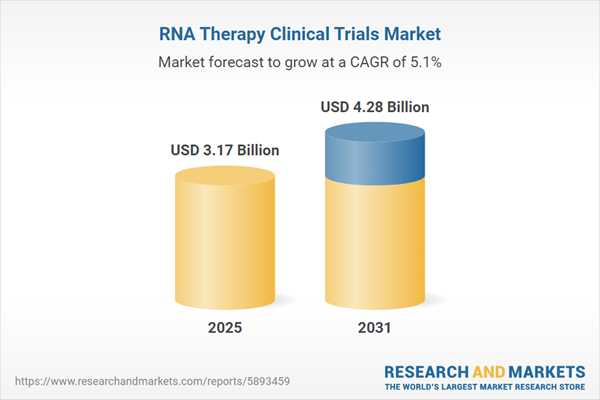
| Estimated Market Value ( USD | $ 3.17 Billion |
| Forecasted Market Value ( USD | $ 4.28 Billion |
| Compound Annual Growth Rate | 5.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |