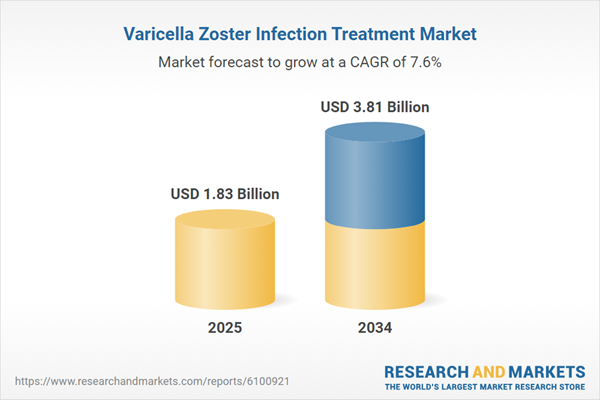
Varicella Zoster Infection Treatment Market Overview
The varicella-zoster virus, also called human herpesvirus 3, leads to chickenpox in kids and shingles in adults. The treatment for current Varicella Zoster infection consists of antiviral medications such as acyclovir, valacyclovir, or famciclovir, in addition to pain relievers and corticosteroids. There are preventive vaccines for Varicella Zoster Infection that is due to the varicella-zoster virus and causes both chickenpox and shingles. The advances in technology and the rising understanding of viral latency and reactivation are driving the growth of the market.Varicella Zoster Infection Treatment Market Growth Drivers
Increasing Disease Prevalence and Surveillance Advancements
The higher awareness and prevalence of new virus variants are driving the demand for varicella zoster infection treatment. For instance, in September 2023, the discovery of the Clade 9 variant in India which is common in countries like Germany, the UK, and the US, highlighted the need for improved surveillance and treatment options. Such new strains are expected to increase demand for treatment and drive market growth during the forecast period.Varicella Zoster Infection Treatment Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Increasing Emphasis on Vaccination
There is an increased focus on managing infection rates with vaccination. For instance, The Centers for Disease Control and Prevention recommended two doses of Shingrix for adults over 50 and those with weakened immune systems, aiming to prevent Herpes zoster and complications.Emergence of New Viral Strains
The discovery of the Clade 9 variant of the varicella-zoster virus in India indicated an increasing importance in the treatment due to the emergence of new viral strains. With the emergence of new strains, healthcare professionals are increasing continuous monitoring and investigation which are essential for adjusting to these changes.Rising Need to Reduce the Risk of Severe Complications
The market is witnessing an increased focus on preventing severe complications associated with varicella zoster infections, such as postherpetic neuralgia and encephalitis. With the aging population and individuals with weakened immune systems being more vulnerable to these conditions, the treatment market is expected to witness significant growth in the forecast period.Growing Need for Specialized Treatments
The increasing demand for specialized treatment such as therapies tailored to the unique needs of high-risk populations, such as the immunocompromised individuals is a major market trend. The focus on specialized antiviral treatments, personalized dosages, and a deeper understanding is contributing to the development of more effective and safer therapies.Varicella Zoster Infection Treatment Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Treatment:
- Vaccines
- Medication
- Others
Market Breakup by Route of Administration:
- Oral
- Topical
- Parenteral
Market Breakup by End User:
- Hospitals
- Homecare
- Specialty Clinics
- Others
Market Breakup by Region:
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Varicella Zoster Infection Treatment Market Share
Segmentation Based on Treatment to Witness Substantial Growth
Based on the treatment, the market is segmented into vaccines, medication, and others. Vaccines are expected to dominate the market in the forecast period as they play a crucial role in the treatment by offering preventative defense against chickenpox. Antiviral medications address ongoing infections, while vaccines focus on prevention to achieve herd immunity and lower healthcare expenses.Varicella Zoster Infection Treatment Market Analysis by Region
The market is divided into regions such as the United States, EU-4 (Germany, France, Italy, Spain), the United Kingdom, Japan, and India. The United States is expected to lead the market share due to growing cases of chickenpox and shingles among older adults and immunocompromised individuals. Additionally, the region focuses on vaccination and progress in antiviral treatments such as Acyclovir and Shingrix are crucial in preventing these infections.Leading Players in the Varicella Zoster Infection Treatment Market
The key features of the market report comprise patent analysis, clinical trial analysis, grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies in the market are as follows:Merck & Co., Inc
Merck & Co., Inc., an American pharmaceutical company based in New Jersey, offers VARIVAX® for varicella zoster infection treatment. The vaccine protects against chickenpox through active immunization in individuals aged 12 months and older.GSK plc
GlaxoSmithKline, a British pharmaceutical company, launched the Shingrix vaccine in India in April 2023 for treating shingles caused by the varicella-zoster virus. The vaccine provides over 90% efficacy and long-lasting protection.Pfizer Inc
Pfizer Inc., an American pharmaceutical corporation headquartered in Manhattan, New York City, is a major market player. The company is collaborating with BioNTech to develop an mRNA-based shingles vaccine.F. Hoffmann-La Roche AG
Roche, a Swiss multinational healthcare company, offers the UC-TIB-HSV/VZV real-time PCR kit for diagnosing herpes simplex virus and varicella zoster virus infections. This diagnostic tool aids in accurate detection and management of infections in transplant and immunocompromised patients.Other key players in the market include Sanofi S.A., Novartis AG, Viatris Inc., and Takeda Pharmaceutical Company.
Key Questions Answered in the Varicella Zoster Infection Treatment Market Report
- What was the varicella zoster infection treatment market value in 2024?
- What is the varicella zoster infection treatment market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is the market segmentation based on treatment?
- What is the market segmentation based on the route of administration?
- What is the market breakup based on the end user?
- What major factors aid the demand for varicella zoster infection treatment?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the major drivers, opportunities, and restraints in the market?
- What are the major trends influencing the market?
- Which regional market is expected to dominate the market share in the forecast period?
- Which country is likely to experience elevated growth during the forecast period?
- Who are the key players involved in the varicella zoster infection treatment market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Merck & Co., Inc.
- GSK plc
- Pfizer Inc.
- F. Hoffmann-La Roche AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 1.83 Billion |
| Forecasted Market Value ( USD | $ 3.81 Billion |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |