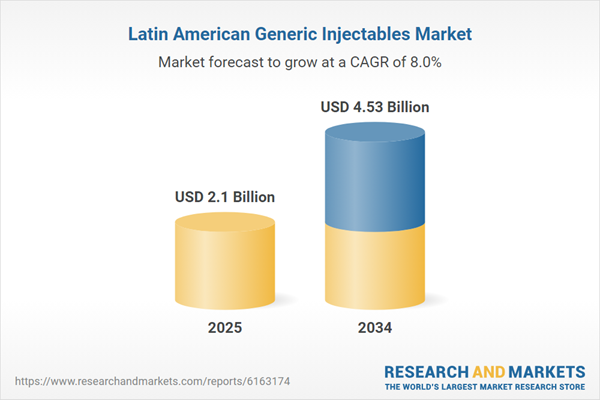
Latin America Generic Injectables Market Analysis
Generic injectables are similar to branded injectable medications in terms of active ingredients, strength, and clinical performance. However, they are typically sold at a lower cost and thus experience increased government and regulatory support for their manufacture and market penetration. The growing burden of chronic diseases and the increasing aging population are contributing to the expansion of the global generic injectables market. Further, the surge in the demand for cost-effective medications to address the rising healthcare needs is driving the Latin America generic injectables market growth.In March 2023, GlaxoSmithKline (GSK) signed deals with three companies (Aurobindo Pharma, Cipla, and Viatris) that will allow them to manufacture cost-effective generic versions of its long-acting HIV preventive medicine (injectable drug cabotegravir) with a supply in 90 countries, primarily in lower-income countries with a high prevalence of HIV. GSK also announced a program with the Medicines Patent Pool (a United Nations-backed healthcare organization) in July 2022, with the aim to offer accelerated access to new HIV therapies in poor countries. Through this program, the company claims to introduce the generic form of its injection by 2026. The rise in such initiatives to combat drug shortages and address the need for affordable life-saving medications is poised to fuel the Latin America generic injectables market demand.
The patent expiration of brand-name injectables stimulates the entry of generic versions which directly impacts the market dynamics signfficantly. For instance, the patent of Danish firm Novo Nordisk's popular diabetes and weight-loss subcutaneous injectable drug, semaglutide, will expire in 2026 in Brazil. In May 2023, the Brazilian federal court denied semaglutide patent extension request by the company, which means that the injectable formulations of semaglutide, Wegovy and Ozempic, will experience competition from first generics just after two years from now. Thus, the expiration of market exclusivity of original injectables is projected to elevate the market value in the forecast period.
Latin America Generic Injectables Market Segmentation
The report offers a detailed analysis of the market based on the following segments:Product Type
- Large Molecule Injectables
- Monoclonal Antibodies (mAbs)
- Insulin
- Others
- Small Molecule Injectables
Market Breakup by Container Type
- Vials
- Premix
- Prefilled Syringes
- Ampoules
- Others
Market Breakup by Application
- Oncology
- Cardiovascular
- CNS
- Infectious Diseases
- Autoimmune Disorders
- Others
Market Breakup by Route of Administration
- Intravenous
- Intramuscular
- Subcutaneous
- Others
Market Breakup by Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Others
Market Breakup by Region
- Brazil
- Argentina
- Mexico
- Others
Leading Players in the Latin America Generic Injectables Market
The key features of the market report include patent analysis, grants analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:Pfizer Inc.
Pfizer has a flourishing business in the sterile injectables category, offering both generic and branded products. It acquired Hospira, the world's leading provider of injectable drugs and infusion technologies, in September 2015, to reinforce its position in the generic injectables market.Viatris Inc.
This American global pharmaceutical and healthcare corporation is one of the largest generic drug manufacturers in the world, specializing in developing complex injectables across a broad range of therapeutic areas.Biocon
Biocon is a fully integrated biopharmaceutical company that develops complex generic formulations and active pharmaceutical ingredients (APIs).Lupin
Lupin Limited ranks as one of the largest generic pharmaceutical companies by revenue globally. Recently, it received regulatory approval in the United States to market a generic drug to treat bacterial infections.Other players in the market include Aurobindo Pharma Limited and Sun Pharmaceutical Industries Ltd.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Pfizer Inc.
- Viatris Inc.
- Biocon
- Lupin.
- Aurobindo Pharma Limited
- Sun Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 2.1 Billion |
| Forecasted Market Value ( USD | $ 4.53 Billion |
| Compound Annual Growth Rate | 8.0% |
| Regions Covered | Latin America |
| No. of Companies Mentioned | 6 |